Overexpression of CBX3 in Pancreatic Adenocarcinoma Promotes Cell Cycle Transition-Associated Tumor Progression
- PMID: 29903985
- PMCID: PMC6032220
- DOI: 10.3390/ijms19061768
Overexpression of CBX3 in Pancreatic Adenocarcinoma Promotes Cell Cycle Transition-Associated Tumor Progression
Abstract
Background: Previous studies showed that Chromobox protein homolog 3 (CBX3) was overexpressed in several types of human cancers, however its pattern and role in pancreatic adenocarcinoma (PAAD) has not yet been understood. The aim of this study was to identify the expression and function of CBX3 in PAAD.
Methods: Data of transcriptomic and protein expression of CBX3 in PAAD were collected from different databases and analyzed. The in vitro and in vivo role of CBX3 in PAAD was examined.
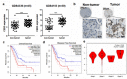
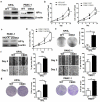
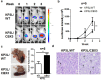
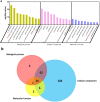
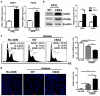
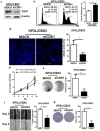
Results: CBX3 was overexpressed in human PAAD tissues, which was associated with poor prognosis of overall and disease-free survival of the patients. Overexpression of CBX3 induced the in vitro proliferation, anchorage-free growth, migration and invasion of the PAAD cells, and led to in vivo growth of orthotoptic PAAD tumors in mice. GO and KEGG pathway analysis, as well as experimental observation showed that CBX3 may be associated with cell cycle transition of PAAD cells, and cyclin-dependent kinase 1 (CDK1) and proliferating cell nuclear antigen (PCNA) may mediate the tumor-promoting action of CBX3. CDK1 knockdown attenuated the cell cycle transition, proliferation and invasion of CBX3-overexpressing PAAD cells.
Conclusion: Our findings suggest the tumor-promoting role of CBX3 in PAAD to be targeted by novel therapeutic strategies.
Keywords: CBX3; CDK1; cell cycle regulation; pancreatic adenocarcinoma; tumor progression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

