Farrerol Relieve Lipopolysaccharide (LPS)-Induced Mastitis by Inhibiting AKT/NF-κB p65, ERK1/2 and P38 Signaling Pathway
- PMID: 29904013
- PMCID: PMC6032361
- DOI: 10.3390/ijms19061770
Farrerol Relieve Lipopolysaccharide (LPS)-Induced Mastitis by Inhibiting AKT/NF-κB p65, ERK1/2 and P38 Signaling Pathway
Abstract
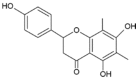
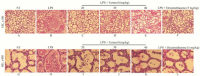
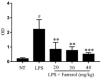
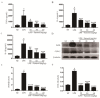
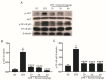
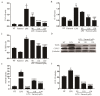
Farrerol has been proved to have an anti-inflammatory effect. However, the effects of farrerol on mastitis have not been investigated. This study was aimed to investigate the effect and mechanism of farrerol in lipopolysaccharide (LPS)-induced mouse mastitis and LPS-induced inflammatory response of mouse mammary epithelial cells (mMECs). In vivo, LPS were injected to the tetrad pair of nipples for establishing mouse mastitis, and then tested the effect of farrerol on histopathological changes, inflammatory response and activation degree of protein kinase B (AKT), nuclear factor-kappa B p65 (NF-κB p65), p38, extracellular regulated protein kinase (ERK1/2). In vitro, the mMECs were incubated by farrerol for 1 h following by stimulating with LPS, and then the inflammatory response and the related signaling pathways were detected. The in vivo results found that farrerol could improve pathological injury of mammary gland, attenuate the activity of myeloperoxidase (MPO), inhibit the production of pro-inflammatory mediators and the phosphorylation of AKT, NF-κB p65, p38 and ERK1/2. The in vitro results also found farrerol inhibited inflammatory response and the related signaling pathways. Collectively, this study revealed that farrerol inhibits the further development of LPS-induced mastitis by inhibiting inflammatory response via down regulating phosphorylation of AKT, NF-κB p65, p38, and ERK1/2. These findings suggest that farrerol may be used as an anti-inflammatory drug for mastitis.
Keywords: LPS; Mitogen-activated protein kinase (MAPK); NF-κB; farrerol; mastitis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Evodiamine Relieve LPS-Induced Mastitis by Inhibiting AKT/NF-κB p65 and MAPK Signaling Pathways.Inflammation. 2022 Feb;45(1):129-142. doi: 10.1007/s10753-021-01533-9. Epub 2021 Aug 16. Inflammation. 2022. PMID: 34401976
-
Peiminine Protects against Lipopolysaccharide-Induced Mastitis by Inhibiting the AKT/NF-κB, ERK1/2 and p38 Signaling Pathways.Int J Mol Sci. 2018 Sep 6;19(9):2637. doi: 10.3390/ijms19092637. Int J Mol Sci. 2018. PMID: 30200569 Free PMC article.
-
Morin alleviates LPS-induced mastitis by inhibiting the PI3K/AKT, MAPK, NF-κB and NLRP3 signaling pathway and protecting the integrity of blood-milk barrier.Int Immunopharmacol. 2020 Jan;78:105972. doi: 10.1016/j.intimp.2019.105972. Epub 2019 Nov 8. Int Immunopharmacol. 2020. PMID: 31711938
-
The pharmacological properties and corresponding mechanisms of farrerol: a comprehensive review.Pharm Biol. 2022 Dec;60(1):9-16. doi: 10.1080/13880209.2021.2006723. Pharm Biol. 2022. PMID: 34846222 Free PMC article.
-
Overview of Research Development on the Role of NF-κB Signaling in Mastitis.Animals (Basel). 2020 Sep 10;10(9):1625. doi: 10.3390/ani10091625. Animals (Basel). 2020. PMID: 32927884 Free PMC article. Review.
Cited by
-
Nanocomposite Hydrogel for Real-Time Wound Status Monitoring and Comprehensive Treatment.Adv Sci (Weinh). 2024 Nov;11(42):e2405924. doi: 10.1002/advs.202405924. Epub 2024 Sep 13. Adv Sci (Weinh). 2024. PMID: 39269428 Free PMC article.
-
Tectorigenin inhibits inflammatory responses in murine inflammatory bowel disease and LPS-stimulated macrophages via inactivating MAPK signaling pathway.Immun Inflamm Dis. 2024 May;12(5):e1077. doi: 10.1002/iid3.1077. Immun Inflamm Dis. 2024. PMID: 38722267 Free PMC article.
-
Anti‑inflammatory activity of 3,5,6,7,3',4'‑hexamethoxyflavone via repression of the NF‑κB and MAPK signaling pathways in LPS‑stimulated RAW264.7 cells.Mol Med Rep. 2020 Sep;22(3):1985-1993. doi: 10.3892/mmr.2020.11252. Epub 2020 Jun 18. Mol Med Rep. 2020. PMID: 32705181 Free PMC article.
-
Wogonin attenuates inflammation and oxidative stress in lipopolysaccharide-induced mastitis by inhibiting Akt/NF-κB pathway and activating the Nrf2/HO-1 signaling.Cell Stress Chaperones. 2023 Nov;28(6):989-999. doi: 10.1007/s12192-023-01391-4. Epub 2023 Nov 1. Cell Stress Chaperones. 2023. PMID: 37910344 Free PMC article.
-
Expression and role of miR-338-3p in peripheral blood and placenta of patients with pregnancy-induced hypertension.Exp Ther Med. 2020 Jul;20(1):418-426. doi: 10.3892/etm.2020.8719. Epub 2020 May 6. Exp Ther Med. 2020. PMID: 32537006 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

