Novel Polyethers from Screening Actinoallomurus spp
- PMID: 29904034
- PMCID: PMC6023020
- DOI: 10.3390/antibiotics7020047
Novel Polyethers from Screening Actinoallomurus spp
Abstract
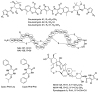
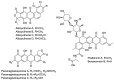
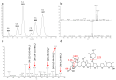
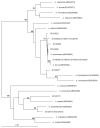
In screening for novel antibiotics, an attractive element of novelty can be represented by screening previously underexplored groups of microorganisms. We report the results of screening 200 strains belonging to the actinobacterial genus Actinoallomurus for their production of antibacterial compounds. When grown under just one condition, about half of the strains produced an extract that was able to inhibit growth of Staphylococcus aureus. We report here on the metabolites produced by 37 strains. In addition to previously reported aminocoumarins, lantibiotics and aromatic polyketides, we described two novel and structurally unrelated polyethers, designated α-770 and α-823. While we identified only one producer strain of the former polyether, 10 independent Actinoallomurus isolates were found to produce α-823, with the same molecule as main congener. Remarkably, production of α-823 was associated with a common lineage within Actinoallomurus, which includes A.fulvus and A.amamiensis. All polyether producers were isolated from soil samples collected in tropical parts of the world.
Keywords: Actinoallomurus; antibiotics polyethers; screening.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Donadio S., Busti E., Monciardini P., Bamonte R., Mazza P., Sosio M., Cavaletti L. Sources of polyketides and non-ribosomal peptides. Ernst Schering Res. Found. Workshop. 2005;51:19–41. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

