Parasite motility is critical for virulence of African trypanosomes
- PMID: 29904094
- PMCID: PMC6002391
- DOI: 10.1038/s41598-018-27228-0
Parasite motility is critical for virulence of African trypanosomes
Abstract
African trypanosomes, Trypanosoma brucei spp., are lethal pathogens that cause substantial human suffering and limit economic development in some of the world's most impoverished regions. The name Trypanosoma ("auger cell") derives from the parasite's distinctive motility, which is driven by a single flagellum. However, despite decades of study, a requirement for trypanosome motility in mammalian host infection has not been established. LC1 is a conserved dynein subunit required for flagellar motility. Prior studies with a conditional RNAi-based LC1 mutant, RNAi-K/R, revealed that parasites with defective motility could infect mice. However, RNAi-K/R retained residual expression of wild-type LC1 and residual motility, thus precluding definitive interpretation. To overcome these limitations, here we generate constitutive mutants in which both LC1 alleles are replaced with mutant versions. These double knock-in mutants show reduced motility compared to RNAi-K/R and are viable in culture, but are unable to maintain bloodstream infection in mice. The virulence defect is independent of infection route but dependent on an intact host immune system. By comparing different mutants, we also reveal a critical dependence on the LC1 N-terminus for motility and virulence. Our findings demonstrate that trypanosome motility is critical for establishment and maintenance of bloodstream infection, implicating dynein-dependent flagellar motility as a potential drug target.
Conflict of interest statement
The authors declare no competing interests.
Figures

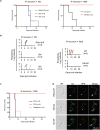

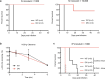
References
-
- Custodio, H. Protozoan Parasites. Pediatr. Rev. 37, 59–69; quiz 70–51, 10.1542/pir.2015-0006 (2016). - PubMed
-
- Hotez PJ, Dumonteil E, Heffernan MJ, Bottazzi ME. Innovation for the ‘bottom 100 million’: eliminating neglected tropical diseases in the Americas. Adv. Exp. Med. Biol. 2013;764:1–12. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

