An interdisciplinary consensus on the management of bone metastases from renal cell carcinoma
- PMID: 29904105
- PMCID: PMC7136176
- DOI: 10.1038/s41585-018-0034-9
An interdisciplinary consensus on the management of bone metastases from renal cell carcinoma
Abstract
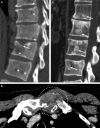
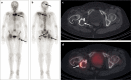
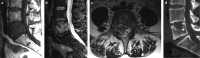
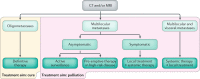
Bone is a major site of haematogenous tumour cell spread in renal cell carcinoma (RCC), and most patients with RCC will develop painful and functionally disabling bone metastases at advanced disease stages. The prognosis of these patients is generally poor and the treatment is, therefore, aimed at palliation. However, RCC-associated bone metastases can be curable in select patients. Current data support a multimodal management strategy that includes wide resection of lesions, radiotherapy, systemic therapy, and other local treatment options, which can improve quality of life and survival. Nevertheless, the optimal approach for metastatic bone disease in RCC has not yet been defined and practical recommendations are rare. To improve the management and outcomes of patients with RCC and bone metastases, the International Kidney Cancer Coalition and the interdisciplinary working group on renal tumours of the German Cancer Society convened a meeting of experts with a global perspective to perform an unstructured review and elaborate on current treatment strategies on the basis of published data and expertise. The panel formulated recommendations for the diagnosis and treatment of patients with RCC and metastasis to the bone. Furthermore, the experts summarized current challenges and unmet patient needs that should be addressed in the future.
Conflict of interest statement
V.G. has participated in advisory boards for Bristol Myer Squibb (BMS), Eisai, Ipsen, Novartis, Pfizer, and Roche, has received honoraria for compensation from BMS, Eisai, Ipsen, Novartis, Pfizer, and Roche, and has received travel grants from BMS, MSD Merck, Novartis, and Pfizer. B.E. has received honoraria and/or travel grants from Pfizer. Uronauten receives no grants from the pharmaceutical industry. A.B. has participated in advisory boards for BMS, Ipsen, Novartis, Pfizer, and Roche/Genentech. A.F. has received honoraria and/or travel grants from Bayer, Ipsen, and Pfizer. R.H.G. has participated in advisory boards for Ipsen and Pfizer and has received travel grants from Ipsen and Pfizer. A.G. has received honoraria and/or travel grants from Bayer, Novartis, and Pfizer. M.S. participated in advisory boards for AVEO, BMS, Eisai, EUSA Pharma, Exelixis, Ipsen, Novartis, Peloton, Pfizer, and Roche/Genentech, has received travel grants from Bayer, BMS, Eisai, Ipsen, Novartis, and Pfizer, and has received research grants from AVEO, BMS, Eisai, Exelixis, Ipsen, Novartis, Pfizer, and Roche/Genentech. The International Kidney Cancer Coalition (IKCC) receives grants from Global Offices of Ipsen/Exelixis, Merck/MDS, BMS, Pfizer, Novartis, and Eisai. T.G., T.D., M.P., H.R.D., K.A.G., C.v.F., and A.M. declare no competing interests.
Figures
References
-
- Adiga GU, et al. Characterization of bone metastases in patients with renal cell cancer. BJU Int. 2004;93:1237–1240. - PubMed
-
- Wood SL, Brown JE. Skeletal metastasis in renal cell carcinoma: current and future management options. Cancer Treat. Rev. 2012;38:284–291. - PubMed
-
- Woodward E, et al. Skeletal complications and survival in renal cancer patients with bone metastases. Bone. 2011;48:160–166. - PubMed
-
- Weber K, Doucet M, Kominsky S. Renal cell carcinoma bone metastasis—elucidating the molecular targets. Cancer Metastasis Rev. 2007;26:691–704. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

