Oxidation of squalene by singlet oxygen and free radicals results in different compositions of squalene monohydroperoxide isomers
- PMID: 29904110
- PMCID: PMC6002538
- DOI: 10.1038/s41598-018-27455-5
Oxidation of squalene by singlet oxygen and free radicals results in different compositions of squalene monohydroperoxide isomers
Abstract
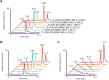
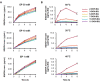
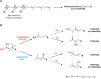
Oxidation of squalene (SQ) causes a decline in the nutritional value of SQ in foods, as well as an accumulation of SQ oxidation products in skin lipids which lead to adverse skin conditions. However, mechanistic insights as to how SQ is oxidized by different oxidation mechanisms have been limited, and thus effective measures towards the prevention of SQ oxidation have not been identified. In this study, we oxidized SQ by either singlet oxygen oxidation or free radical oxidation, and monitored the formation of the six SQ monohydroperoxide (SQOOH) isomers, the primary oxidation products of SQ, at the isomeric level. While singlet oxygen oxidation of SQ resulted in the formation of similar amounts of the six SQOOH isomers, free radical oxidation of SQ mainly formed two types of isomers, 2-OOH-SQ and 3-OOH-SQ. The addition of β-carotene during singlet oxygen oxidation, and the addition of α-tocopherol during free radical oxidation lead to a dose-dependent decrease in the formation of SQOOH isomers. Such results suggest that the analysis of SQOOH at the isomeric level allows for the determination of the cause of SQ oxidation in various samples, and provides a foothold for future studies concerning the prevention of SQ oxidation.
Conflict of interest statement
The authors declare no competing interests.
Figures

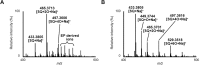


Similar articles
-
Novel Photoinduced Squalene Cyclic Peroxide Identified, Detected, and Quantified in Human Skin Surface Lipids.Antioxidants (Basel). 2021 Nov 4;10(11):1760. doi: 10.3390/antiox10111760. Antioxidants (Basel). 2021. PMID: 34829631 Free PMC article.
-
Evaluation of squalene oxidation mechanisms in human skin surface lipids and shark liver oil supplements.Ann N Y Acad Sci. 2019 Dec;1457(1):158-165. doi: 10.1111/nyas.14219. Epub 2019 Aug 26. Ann N Y Acad Sci. 2019. PMID: 31452205
-
Mass Spectrometric Discrimination of Squalene Monohydroperoxide Isomers.J Oleo Sci. 2017 Mar 1;66(3):227-234. doi: 10.5650/jos.ess16159. Epub 2017 Feb 10. J Oleo Sci. 2017. PMID: 28190805
-
Lipid oxidation in the skin.Free Radic Res. 2015;49(7):827-34. doi: 10.3109/10715762.2014.976213. Epub 2014 Nov 11. Free Radic Res. 2015. PMID: 25312699 Review.
-
An update review on Commiphora molmol and related species.J Egypt Soc Parasitol. 2008 Dec;38(3):763-96. J Egypt Soc Parasitol. 2008. PMID: 19209761 Review.
Cited by
-
Squalene: More than a Step toward Sterols.Antioxidants (Basel). 2020 Aug 2;9(8):688. doi: 10.3390/antiox9080688. Antioxidants (Basel). 2020. PMID: 32748847 Free PMC article. Review.
-
Tetrathienothiophene Porphyrin as a Metal-Free Sensitizer for Room-Temperature Triplet-Triplet Annihilation Upconversion.Front Chem. 2022 Apr 26;10:809863. doi: 10.3389/fchem.2022.809863. eCollection 2022. Front Chem. 2022. PMID: 35559213 Free PMC article.
-
Novel Photoinduced Squalene Cyclic Peroxide Identified, Detected, and Quantified in Human Skin Surface Lipids.Antioxidants (Basel). 2021 Nov 4;10(11):1760. doi: 10.3390/antiox10111760. Antioxidants (Basel). 2021. PMID: 34829631 Free PMC article.
-
Liposomes Loaded with Unsaponifiable Matter from Amaranthus hypochondriacus as a Source of Squalene and Carrying Soybean Lunasin Inhibited Melanoma Cells.Nanomaterials (Basel). 2021 Jul 30;11(8):1960. doi: 10.3390/nano11081960. Nanomaterials (Basel). 2021. PMID: 34443791 Free PMC article.
-
Significance of Singlet Oxygen Molecule in Pathologies.Int J Mol Sci. 2023 Feb 1;24(3):2739. doi: 10.3390/ijms24032739. Int J Mol Sci. 2023. PMID: 36769060 Free PMC article. Review.
References
-
- Yarkoni E, Rapp HJ. Tumor regression after intralesional injection of mycobacterial components emulsified in 2,6,10,15,19,23-hexamethyl-2,6,10,14,18,22-tetracosahexaene (squalene), 2,6,10,15,19,23-hexamethyltetracosane (squalane), peanut oil, or mineral oil. Cancer Res. 1979;39:1518–1520. - PubMed
-
- Nakagawa M, et al. Potentiation by squalene of the cytotoxicity of anticancer agents against cultured mammalian cells and murine tumor. Jpn J Cancer Res. 1985;76:315–320. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

