Pharmacological activation of epidermal growth factor receptor signaling inhibits colitis-associated cancer in mice
- PMID: 29904166
- PMCID: PMC6002410
- DOI: 10.1038/s41598-018-27353-w
Pharmacological activation of epidermal growth factor receptor signaling inhibits colitis-associated cancer in mice
Abstract
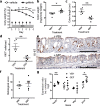
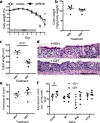
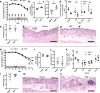
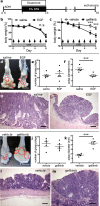
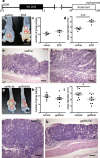
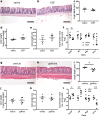
Current treatments for inflammatory bowel disease (IBD) target the overactive immune response of the intestinal mucosa. However, epidermal growth factor (EGF), an activating ligand of the EGF receptor (EGFR), has been shown to induce disease remission through direct targeting of intestinal mucosal healing. Despite promising preclinical and clinical results, this EGFR-activating therapy has not progressed, in part due to the potential for carcinogenesis associated with long-term use and the increased risk of colitis-associated cancer (CAC) in IBD. Here we tested whether pharmacological modulation of EGFR altered outcomes of CAC in the murine azoxymethane/dextran sulfate sodium model. We found that administering EGF during the period of maximum colitis severity ("early"), coincident with the initiation and early promotion of tumors, improved outcomes of colitis and reduced tumor size. In contrast, daily EGF administration beginning ~2 months after tumor initiation ("late") increased tumor size. Administration of the EGFR kinase inhibitor gefitinib increased the tumor size when the drug was given early and decreased the tumor size when the drug was administered late. EGF administration not only reduced colonic cytokine and chemokine expression during injury, but also baseline chemokine expression in homeostasis. These results suggest that EGFR activation during acute bouts of colitis may reduce the long-term burden of CAC.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

