Liver X receptors in lipid signalling and membrane homeostasis
- PMID: 29904174
- PMCID: PMC6433546
- DOI: 10.1038/s41574-018-0037-x
Liver X receptors in lipid signalling and membrane homeostasis
Abstract
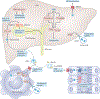
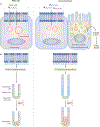
Liver X receptors α and β (LXRα and LXRβ) are nuclear receptors with pivotal roles in the transcriptional control of lipid metabolism. Transcriptional activity of LXRs is induced in response to elevated cellular levels of cholesterol. LXRs bind to and regulate the expression of genes that encode proteins involved in cholesterol absorption, transport, efflux, excretion and conversion to bile acids. The coordinated, tissue-specific actions of the LXR pathway maintain systemic cholesterol homeostasis and regulate immune and inflammatory responses. LXRs also regulate fatty acid metabolism by controlling the lipogenic transcription factor sterol regulatory element-binding protein 1c and regulate genes that encode proteins involved in fatty acid elongation and desaturation. LXRs exert important effects on the metabolism of phospholipids, which, along with cholesterol, are major constituents of cellular membranes. LXR activation preferentially drives the incorporation of polyunsaturated fatty acids into phospholipids by inducing transcription of the remodelling enzyme lysophosphatidylcholine acyltransferase 3. The ability of the LXR pathway to couple cellular sterol levels with the saturation of fatty acids in membrane phospholipids has implications for several physiological processes, including lipoprotein production, dietary lipid absorption and intestinal stem cell proliferation. Understanding how LXRs regulate membrane composition and function might provide new therapeutic insight into diseases associated with dysregulated lipid metabolism, including atherosclerosis, diabetes mellitus and cancer.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures
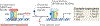

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

