FGF23 and Fetuin-A Interaction in the Liver and in the Circulation
- PMID: 29904273
- PMCID: PMC6001652
- DOI: 10.7150/ijbs.23256
FGF23 and Fetuin-A Interaction in the Liver and in the Circulation
Abstract
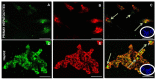
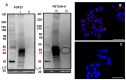
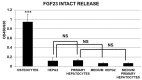
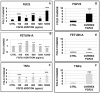
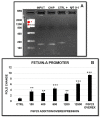
Recently it has been demonstrated that Fetuin-A, an anti-inflammatory protein synthesized by the liver, is produced also in bone by an FGF23-regulated pathway. FGF23 has been also demonstrated to induce inflammatory cytokine production in the liver. This study aimed to explore if FGF23 plays a role in the Fetuin-A production in the liver cells too and the possible relationships with FGF23 pro-inflammatory effects. FGF23 and Fetuin-A were studied in liver, kidney and in plasma with immunochemistry, immunoprecipitation, western blot, chromatin immunoprecipitation, duolink, ELISA, qrtPCR methodology. FGF23 is produced, but not secreted by the liver cells. In hepatocytes and circulation, FGF23 was present only strictly linked to Fetuin-A, while Fetuin-A was found also in unbounded form. No link was observed in the kidney. FGF23 up to 600 pg/ml stimulates, while, at higher concentrations, reduces Fetuin-A expression. Notably, overall the range of concentrations, FGF23 stimulates Fetuin-A promoter, TNFα and IL6 expression. In the nucleus, FGF23 seems to act as a direct transcription factor of Fetuin-A promoter. These results suggest that FGF23 played a direct regulatory role in Fetuin-A expression in liver cells with a biphasic effect: Fetuin-A progressively increases when FGF23 increases up to 400-600 pg/mL, and declines at higher FGF23 concentrations. These results lead us to hypothesize: a) a possible epigenetic post-transcriptional regulation; b) a possible counter-regulatory effect of FGF23 induced inflammatory cytokines (TNFα/ NF-κB mechanism). This study could add an additional key for the interpretation of the possible mechanisms linking FGF23, Fetuin-A and inflammation in CKD patients and suggests a role for FGF23 as transcription factor.
Keywords: Chronic kidney disease; Circulation; FGF23; Fetuin-A; Inflammation; Liver.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
-
- Kaneko I, Tatsumi S, Segawa H, Miyamoto KI. Control of phosphate balance by the kidney and intestine. Clin Exp Nephrol; 2016. Review. - PubMed
-
- Messa P. FGF23 and vascular calcifications: another piece of the puzzle? Nephrol Dial Transplant. 2014;29(8):1447–9. - PubMed
-
- Hasegawa H, Nagano N, Urakawa I, Yamazaki Y, Iijima K, Fujita T. et al. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int. 2010;78(10):975–80. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

