Screening of multimeric β-xylosidases from the gut microbiome of a higher termite, Globitermes brachycerastes
- PMID: 29904275
- PMCID: PMC6001650
- DOI: 10.7150/ijbs.22763
Screening of multimeric β-xylosidases from the gut microbiome of a higher termite, Globitermes brachycerastes
Abstract
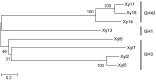
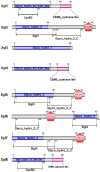
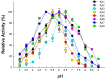
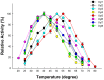
Termite gut microbiome is a rich reservoir for glycoside hydrolases, a suite of enzymes critical for the degradation of lignocellulosic biomass. To search for hemicellulases, we screened 12,000 clones from a fosmid gut library of a higher termite, Globitermes brachycerastes. As a common Southeastern Asian genus, Globitermes distributes predominantly in tropical rain forests and relies on the lignocellulases from themselves and bacterial symbionts to digest wood. In total, 22 positive clones with β-xylosidase activity were isolated, in which 11 representing different restriction fragment length polymorphism (RFLP) patterns were pooled and subjected to 454 pyrosequencing. As a result, eight putative β-xylosidases were cloned and heterologously expressed in Escherichia coli BL21 competent cells. After purification using Ni-NTA affinity chromatography, recombinant G. brachycerastes symbiotic β-xylosidases were characterized enzymatically, including their pH and temperature optimum. In addition to β-xylosidase activity, four of them also exhibited either β-glucosidase or α-arabinosidases activities, suggesting the existence of bifunctional hemicellulases in the gut microbiome of G. brachycerastes. In comparison to multimeric protein engineering, the involvement of naturally occurring multifunctional biocatalysts streamlines the genetic modification procedures and simplifies the overall production processes. Alternatively, these multimeric enzymes could serve as the substitutes for β-glucosidase, β-xylosidase and α-arabinosidase to facilitate a wide range of industrial applications, including food processing, animal feed, environment and waste management, and biomass conversion.
Keywords: Globitermes brachycerastes; fosmid library; glycoside hydrolases; gut microbiome; multimeric hemicellulase; β-xylosidase.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
Similar articles
-
Characterization of a novel salt-, xylose- and alkali-tolerant GH43 bifunctional β-xylosidase/α-l-arabinofuranosidase from the gut bacterial genome.J Biosci Bioeng. 2019 Oct;128(4):429-437. doi: 10.1016/j.jbiosc.2019.03.018. Epub 2019 May 17. J Biosci Bioeng. 2019. PMID: 31109875
-
Analysis of the xynB5 gene encoding a multifunctional GH3-BglX β-glucosidase-β-xylosidase-α-arabinosidase member in Caulobacter crescentus.Antonie Van Leeuwenhoek. 2015 Oct;108(4):993-1007. doi: 10.1007/s10482-015-0552-x. Epub 2015 Aug 12. Antonie Van Leeuwenhoek. 2015. PMID: 26264062
-
Comparison of microbial diversity and carbohydrate-active enzymes in the hindgut of two wood-feeding termites, Globitermes sulphureus (Blattaria: Termitidae) and Coptotermes formosanus (Blattaria: Rhinotermitidae).BMC Microbiol. 2024 Nov 12;24(1):470. doi: 10.1186/s12866-024-03623-8. BMC Microbiol. 2024. PMID: 39533168 Free PMC article.
-
Properties and applications of microbial beta-D-xylosidases featuring the catalytically efficient enzyme from Selenomonas ruminantium.Appl Microbiol Biotechnol. 2010 May;86(6):1647-58. doi: 10.1007/s00253-010-2538-y. Epub 2010 Mar 30. Appl Microbiol Biotechnol. 2010. PMID: 20352422 Review.
-
β-xylosidases and α-L-arabinofuranosidases: accessory enzymes for arabinoxylan degradation.Biotechnol Adv. 2014 Mar-Apr;32(2):316-32. doi: 10.1016/j.biotechadv.2013.11.005. Epub 2013 Nov 15. Biotechnol Adv. 2014. PMID: 24239877 Review.
Cited by
-
Enzymatic hydrolysis of lignocellulosic biomass using a novel, thermotolerant recombinant xylosidase enzyme from Clostridium clariflavum: a potential addition for biofuel industry.RSC Adv. 2022 May 18;12(23):14917-14931. doi: 10.1039/d2ra00304j. eCollection 2022 May 12. RSC Adv. 2022. PMID: 35702232 Free PMC article.
-
Functional metagenomics reveals abundant polysaccharide-degrading gene clusters and cellobiose utilization pathways within gut microbiota of a wood-feeding higher termite.ISME J. 2019 Jan;13(1):104-117. doi: 10.1038/s41396-018-0255-1. Epub 2018 Aug 16. ISME J. 2019. PMID: 30116044 Free PMC article.
-
Characterization of a highly xylose tolerant β-xylosidase isolated from high temperature horse manure compost.BMC Biotechnol. 2021 Oct 24;21(1):61. doi: 10.1186/s12896-021-00722-6. BMC Biotechnol. 2021. PMID: 34689773 Free PMC article.
-
Activity-Based Protein Profiling for the Identification of Novel Carbohydrate-Active Enzymes Involved in Xylan Degradation in the Hyperthermophilic Euryarchaeon Thermococcus sp. Strain 2319x1E.Front Microbiol. 2022 Jan 12;12:734039. doi: 10.3389/fmicb.2021.734039. eCollection 2021. Front Microbiol. 2022. PMID: 35095781 Free PMC article.
-
Xylanolytic Extremozymes Retrieved From Environmental Metagenomes: Characteristics, Genetic Engineering, and Applications.Front Microbiol. 2020 Sep 17;11:551109. doi: 10.3389/fmicb.2020.551109. eCollection 2020. Front Microbiol. 2020. PMID: 33042057 Free PMC article. Review.
References
-
- Aristidou A, Penttilä M. Metabolic engineering applications to renewable resource utilization. Curr Opin Biotechnol. 2000;11:187–198. - PubMed
-
- Bajpai P. Microbial xylanolytic enzyme system: properties and applications. Adv. Appl Microbiol. 1997;43:141–194. - PubMed
-
- McMillan JD. Pretreatment of lignocellulosic biomass. In: Himmel ME, Baker JO, Overend RP, editors. Enzymatic conversion of biomass for fuels production. Washington: American Chemical Society; 1994. pp. 292–324.
-
- Saha BC. Hemicellulose bioconversion. J Indust Microbiol Biotechnol. 2003;30:279–291. - PubMed
-
- Subramaniyan S, Prema P. Biotechnology of microbial xylanases: enzymology, molecular biology and application. Crit Rev Biotechnol. 2002;22:33–64. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

