Medium-Chain Fatty Acids, Beta-Hydroxybutyric Acid and Genetic Modulation of the Carnitine Shuttle Are Protective in a Drosophila Model of ALS Based on TDP-43
- PMID: 29904341
- PMCID: PMC5990617
- DOI: 10.3389/fnmol.2018.00182
Medium-Chain Fatty Acids, Beta-Hydroxybutyric Acid and Genetic Modulation of the Carnitine Shuttle Are Protective in a Drosophila Model of ALS Based on TDP-43
Abstract
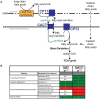
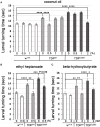
ALS patients exhibit dyslipidemia, hypermetabolism and weight loss; in addition, cellular energetics deficits have been detected prior to denervation. Although evidence that metabolism is altered in ALS is compelling, the mechanisms underlying metabolic dysregulation and the contribution of altered metabolic pathways to disease remain poorly understood. Here we use a Drosophila model of ALS based on TDP-43 that recapitulates hallmark features of the disease including locomotor dysfunction and reduced lifespan. We performed a global, unbiased metabolomic profiling of larvae expressing TDP-43 (wild-type, TDPWT or disease-associated mutant, TDPG298S) and identified several lipid metabolism associated alterations. Among these, we found a significant increase in carnitine conjugated long-chain fatty acids and a significant decrease in carnitine, acetyl-carnitine and beta-hydroxybutyrate, a ketone precursor. Taken together these data suggest a deficit in the function of the carnitine shuttle and reduced lipid beta oxidation. To test this possibility we used a combined genetic and dietary approach in Drosophila. Our findings indicate that components of the carnitine shuttle are misexpressed in the context of TDP-43 proteinopathy and that genetic modulation of CPT1 or CPT2 expression, two core components of the carnitine shuttle, mitigates TDP-43 dependent locomotor dysfunction, in a variant dependent manner. In addition, feeding medium-chain fatty acids or beta-hydroxybutyrate improves locomotor function, consistent with the notion that bypassing the carnitine shuttle deficit is neuroprotective. Taken together, our findings highlight the potential contribution of the carnitine shuttle and lipid beta oxidation in ALS and suggest strategies for therapeutic intervention based on restoring lipid metabolism in motor neurons.
Keywords: TDP-43; amyotrophic lateral sclerosis; beta lipid oxidation; carnitine shuttle; lipid metabolism; metabolomics.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

