Coronary Blood Flow Is Increased in RV Hypertrophy, but the Shape of Normalized Waves Is Preserved Throughout the Arterial Tree
- PMID: 29904356
- PMCID: PMC5990630
- DOI: 10.3389/fphys.2018.00675
Coronary Blood Flow Is Increased in RV Hypertrophy, but the Shape of Normalized Waves Is Preserved Throughout the Arterial Tree
Abstract
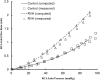
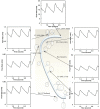
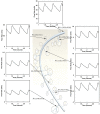
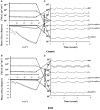
A pulsatile hemodynamic analysis was carried out in the right coronary arterial (RCA) tree of control and RV hypertrophy (RVH) hearts. The shape of flow and wall shear stress (WSS) waves was hypothesized to be maintained throughout the RCA tree in RVH (i.e., similar patterns of normalized flow and WSS waves in vessels of various sizes). Consequently, we reconstructed the entire RCA tree down to the first capillary bifurcation of control and RVH hearts based on measured morphometric data. A Womersley-type model was used to compute the flow and WSS waves in the tree. The hemodynamic parameters obtained from experimental measurements were incorporated into the numerical model. Given an increased number of arterioles, the mean and amplitude of flow waves at the inlet of RCA tree in RVH was found to be two times larger than that in control, but no significant differences (p > 0.05) were found in precapillary arterioles. The increase of stiffness in RCA of RVH preserved the shape of normalized flow and WSS waves, but increased the PWV in coronary arteries and reduced the phase angle difference for the waves between the most proximal RCA and the most distal precapillary arteriole. The study is important for understanding pulsatile coronary blood flow in ventricular hypertrophy.
Keywords: Pulsatile wall shear stress; Womersley-type model; pulsatile flow; right coronary arterial tree; right ventricular hypertrophy.
Figures


References
-
- Butlin M., Qasem A., Battista F., Bozec E., McEniery C. M., Millet-Amaury E., et al. (2013). Carotid-femoral pulse wave velocity assessment using novel cuff-based techniques: comparison with tonometric measurement. J Hypertens. 31, 2237–2243, discussion 2243. 10.1097/HJH.0b013e328363c789 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

