Mapping Determinants of Virus Neutralization and Viral Escape for Rational Design of a Hepatitis C Virus Vaccine
- PMID: 29904384
- PMCID: PMC5991293
- DOI: 10.3389/fimmu.2018.01194
Mapping Determinants of Virus Neutralization and Viral Escape for Rational Design of a Hepatitis C Virus Vaccine
Abstract
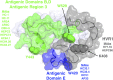
Hepatitis C virus (HCV) continues to spread worldwide with an annual increase of 1.75 million new infections. The number of HCV cases in the U.S. is now greater than the number of HIV cases and is increasing in young adults because of the opioid epidemic sweeping the country. HCV-related liver disease is the leading indication of liver transplantation. An effective vaccine is of paramount importance to control and prevent HCV infection. While this vaccine will need to induce both cellular and humoral immunity, this review is focused on the required antibody responses. For highly variable viruses, such as HCV, isolation and characterization of monoclonal antibodies mediating broad virus neutralization are an important guide for vaccine design. The viral envelope glycoproteins, E1 and E2, are the main targets of these antibodies. Epitopes on the E2 protein have been studied more extensively than epitopes on E1, due to higher antibody targeting that reflects these epitopes having higher degrees of immunogenicity. E2 epitopes are overall organized in discrete clusters of overlapping epitopes that ranged from high conservation to high variability. Other epitopes on E1 and E1E2 also are targets of neutralizing antibodies. Taken together, these regions are important for vaccine design. Another element in vaccine design is based on information on how the virus escapes from broadly neutralizing antibodies. Escape mutations can occur within the epitopes that are involved in antibody binding and in regions that are not involved in their epitopes, but nonetheless reduce the efficiency of neutralizing antibodies. An understanding on the specificities of a protective B cell response, the molecular locations of these epitopes on E1, E2, and E1E2, and the mechanisms, which enable the virus to negatively modulate neutralizing antibody responses to these regions will provide the necessary guidance for vaccine design.
Keywords: antigenic domains; epitopes; hepatitis C virus; human monoclonal antibodies; vaccine design; virus neutralization.
Figures
References
-
- WHO. Global Hepatitis Report, 2017. Geneva: World Health Organization; (2017). 83 p.
-
- Centers for Disease Control and Prevention. New Hepatitis C Infections Nearly Tripled Over Five Years. (2017).
-
- Zibbell JE, Asher AK, Patel RC, Kupronis B, Iqbal K, Ward JW, et al. Increases in acute hepatitis C virus infection related to a growing opioid epidemic and associated injection drug use, United States, 2004 to 2014. Am J Public Health (2018) 108:175–81. 10.2105/AJPH.2017.304132 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

