Thymoquinone reduces spinal cord injury by inhibiting inflammatory response, oxidative stress and apoptosis via PPAR-γ and PI3K/Akt pathways
- PMID: 29904397
- PMCID: PMC5996697
- DOI: 10.3892/etm.2018.6072
Thymoquinone reduces spinal cord injury by inhibiting inflammatory response, oxidative stress and apoptosis via PPAR-γ and PI3K/Akt pathways
Abstract
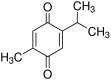
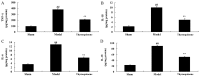
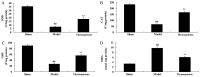
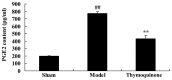
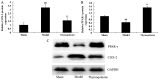
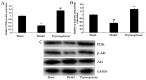
The present study used a mild contusion injury in rat spinal cord to determine that thymoquinone reduces inflammatory response, oxidative stress and apoptosis in a spinal cord injury (SCI) rat model and to demonstrate its possible molecular mechanisms. The rats in the thymoquinone group received 30 mg/kg thymoquinone once daily by intragastric administration from 3 weeks after surgery. Hematoxylin and eosin staining, Basso, Beattie and Bresnahan (BBB) scale and tissue water content detection were used in the present study to analyze the effect of thymoquinone on SCI. The activity of inflammatory response mediators, oxidative stress factors and caspase-3/9 was measured using ELISA kits. Furthermore, western blotting was performed to analyzed the protein expression levels of prostaglandin E2, suppressed cyclooxygenase-2 (COX-2) and activated peroxisome proliferator-activated receptor γ (PPAR-γ), PI3K and Akt. The results from the study demonstrated that thymoquinone increased Basso, Beattie and Bresnahan score and decreased water content in spinal cord tissue. Treatment with thymoquinone decreased inflammatory response [measured by levels of tumor necrosis factor α, interleukin (IL)-1β, IL-6 and IL-18], oxidative stress (measured by levels of superoxide dismutase, catalase, glutathione and malondialdehyde) and cell apoptosis (measured by levels of caspase-3 and caspase-9) in SCI rats. Thymoquinone treatment inhibited prostaglandin E2 activity, suppressed COX-2 protein expression and activated PPAR-γ, PI3K and p-Akt protein expression in SCI rats. These data revealed that thymoquinone reduces inflammatory response, oxidative stress and apoptosis via PPAR-γ and PI3K/Akt pathways in an SCI rat model.
Keywords: peroxisome proliferator-activated receptor γ; phosphoinositide 3-kinase/Akt; spinal cord injury; thymoquinone.
Figures

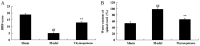
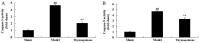
References
-
- Arora M, Harvey LA, Hayes AJ, Chhabra HS, Glinsky JV, Cameron ID, Lavrencic L, Arumugam N, Hossain S, Bedi PK. Effectiveness and cost-effectiveness of telephone-based support versus usual care for treatment of pressure ulcers in people with spinal cord injury in low-income and middle-income countries: Study protocol for a 12-week randomised controlled trial. BMJ Open. 2015;5:e008369. doi: 10.1136/bmjopen-2015-008369. - DOI - PMC - PubMed
-
- Lόpez-Larraz E, Antelis JM, Montesano L, Gil-Agudo A, Minguez J. Continuous decoding of motor attempt and motor imagery from EEG activity in spinal cord injury patients. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:1798–1801. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
