Clindamycin phosphate 1.2%/benzoyl peroxide 3% fixed-dose combination gel versus topical combination therapy of adapalene 0.1% gel and clindamycin phosphate 1.2% gel in the treatment of acne vulgaris in Japanese patients: A multicenter, randomized, investigator-blind, parallel-group study
- PMID: 29905384
- PMCID: PMC6099304
- DOI: 10.1111/1346-8138.14497
Clindamycin phosphate 1.2%/benzoyl peroxide 3% fixed-dose combination gel versus topical combination therapy of adapalene 0.1% gel and clindamycin phosphate 1.2% gel in the treatment of acne vulgaris in Japanese patients: A multicenter, randomized, investigator-blind, parallel-group study
Abstract
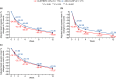
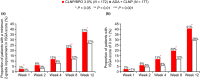
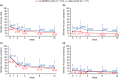
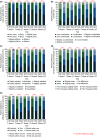
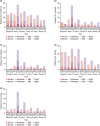
Adapalene 0.1% (ADA) with clindamycin phosphate 1.2% (CLNP; ADA + CLNP) and the fixed-dose combination containing CLNP and benzoyl peroxide 3% (CLNP/BPO 3%) are strongly recommended for the early treatment of acne vulgaris in Japan. Here, we compare the early efficacy and safety of CLNP/BPO 3% with Japanese standard topical use of ADA + CLNP in the treatment of acne vulgaris. In this phase IV, multicenter study, 351 patients were randomized to receive CLNP/BPO 3% or ADA + CLNP for 12 weeks. The primary end-point was percentage change from baseline in total lesion (TL) counts at week 2. Secondary end-points included the percentage change from baseline in TL, inflammatory and non-inflammatory lesion (IL and non-IL) counts, Investigator's Static Global Assessment (ISGA), quality of life (QoL [Skindex-16]) and patient preference. Local tolerability scores and adverse events were also recorded. CLNP/BPO 3% provided a significantly greater percentage reduction from baseline in TL compared with ADA + CLNP at week 2, and week 4. Compared with ADA + CLNP, CLNP/BPO 3% was superior at reducing IL (but not non-IL) over weeks 2-12, was more effective at improving patient QoL and ISGA, and scored higher in patient-preference assessments. Both treatments were well tolerated; adverse drug reactions occurred more frequently in patients receiving ADA + CLNP (37%) than in those receiving CLNP/BPO 3% (17%). In conclusion, CLNP/BPO 3% showed greater efficacy for the early treatment of acne vulgaris in Japan, with a more favorable safety profile compared with ADA + CLNP.
Keywords: acne vulgaris; adapalene; benzoyl peroxide; clindamycin; drug combinations.
© 2018 The Authors. The Journal of Dermatology published by John Wiley & Sons Australia, Ltd on behalf of Japanese Dermatological Association.
Figures
References
-
- Tan JKL, Bhate K. A global perspective on the epidemiology of acne. Br J Dermatol 2015; 172: 3–12. - PubMed
-
- Gollnick HPM. From new findings in acne pathogenesis to new approaches in treatment. J Eur Acad Dermatol Venereol 2015; 29: 1–7. - PubMed
-
- Hayashi N, Akamatsu H, Iwatsuki K. Guideline for the treatment of acne vulgaris. (in Japanese). Jpn J Dermatol 2008; 118(10): 1893–1923.
-
- Hayashi N, Akamatsu H, Iwatsuki K. Guideline for the treatment of acne vulgaris (in Japanese). Jpn J Dermatol 2016; 2016(126): 1045–1086.
-
- Kawashima M, Hashimoto H, Alió Sáenz AB, Ono M, Yamada M. Clindamycin phosphate 1.2%–benzoyl peroxide 3.0% fixed‐dose combination gel has an effective and acceptable safety and tolerability profile for the treatment of acne vulgaris in Japanese patients: a phase III, multicentre, randomised, single‐blinded, active‐controlled, parallel‐group study. Br J Dermatol 2015; 172(2): 494–503. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

