Nrf2 Deficiency Exacerbates Obesity-Induced Oxidative Stress, Neurovascular Dysfunction, Blood-Brain Barrier Disruption, Neuroinflammation, Amyloidogenic Gene Expression, and Cognitive Decline in Mice, Mimicking the Aging Phenotype
- PMID: 29905772
- PMCID: PMC6001893
- DOI: 10.1093/gerona/glx177
Nrf2 Deficiency Exacerbates Obesity-Induced Oxidative Stress, Neurovascular Dysfunction, Blood-Brain Barrier Disruption, Neuroinflammation, Amyloidogenic Gene Expression, and Cognitive Decline in Mice, Mimicking the Aging Phenotype
Abstract
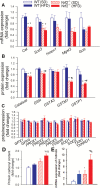
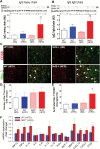
Obesity has deleterious effects on cognitive function in the elderly adults. In mice, aging exacerbates obesity-induced oxidative stress, microvascular dysfunction, blood-brain barrier (BBB) disruption, and neuroinflammation, which compromise cognitive health. However, the specific mechanisms through which aging and obesity interact to remain elusive. Previously, we have shown that Nrf2 signaling plays a critical role in microvascular resilience to obesity and that aging is associated with progressive Nrf2 dysfunction, promoting microvascular impairment. To test the hypothesis that Nrf2 deficiency exacerbates cerebromicrovascular dysfunction induced by obesity Nrf2+/+ and Nrf2-/-, mice were fed an adipogenic high-fat diet (HFD). Nrf2 deficiency significantly exacerbated HFD-induced oxidative stress and cellular senescence, impairment of neurovascular coupling responses, BBB disruption, and microglia activation, mimicking the aging phenotype. Obesity in Nrf2-/- mice elicited complex alterations in the amyloidogenic gene expression profile, including upregulation of amyloid precursor protein. Nrf2 deficiency and obesity additively reduced long-term potentiation in the CA1 area of the hippocampus. Collectively, Nrf2 dysfunction exacerbates the deleterious effects of obesity, compromising cerebromicrovascular and brain health by impairing neurovascular coupling mechanisms, BBB integrity and synaptic function and promoting neuroinflammation. These results support a possible role for age-related Nrf2 dysfunction in the pathogenesis of vascular cognitive impairment and Alzheimer's disease.
Figures


References
-
- Wolf PA, Beiser A, Elias MF, Au R, Vasan RS, Seshadri S. Relation of obesity to cognitive function: importance of central obesity and synergistic influence of concomitant hypertension. The Framingham Heart Study. Curr Alzheimer Res. 2007;4:111–116. - PubMed
-
- Gustafson DR, Karlsson C, Skoog I, Rosengren L, Lissner L, Blennow K. Mid-life adiposity factors relate to blood-brain barrier integrity in late life. J Intern Med. 2007;262:643–650. doi:10.1111/j.1365-2796.2007.01869.x - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG055395/AG/NIA NIH HHS/United States
- R01 AG047879/AG/NIA NIH HHS/United States
- P30 AG050911/AG/NIA NIH HHS/United States
- R01 NS056218/NS/NINDS NIH HHS/United States
- R01 NS100782/NS/NINDS NIH HHS/United States
- U54 GM104938/GM/NIGMS NIH HHS/United States
- R01 HL132553/HL/NHLBI NIH HHS/United States
- R01 EY019494/EY/NEI NIH HHS/United States
- P20 GM103447/GM/NIGMS NIH HHS/United States
- P30 EY021725/EY/NEI NIH HHS/United States
- P20 GM104934/GM/NIGMS NIH HHS/United States
- P30 CA225520/CA/NCI NIH HHS/United States
- T32 AG052363/AG/NIA NIH HHS/United States
- R01 AT006526/AT/NCCIH NIH HHS/United States
- R01 AG038747/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

