Extended use of the wearable cardioverter-defibrillator in patients at risk for sudden cardiac death
- PMID: 29905788
- PMCID: PMC6140450
- DOI: 10.1093/europace/euy091
Extended use of the wearable cardioverter-defibrillator in patients at risk for sudden cardiac death
Abstract
Aims: Data on outcomes in patients using the wearable cardioverter-defibrillator (WCD) > 90 days are limited. We aimed to analyse the clinical course of patients with WCD use ≤90 days vs. WCD use >90 days.
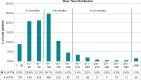
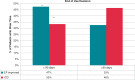
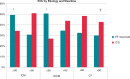
Methods and results: We assessed arrhythmia events during WCD use, and ejection fraction (EF) improvement/implantable cardioverter-defibrillator (ICD) implantation at the end of WCD use in patients with WCD use ≤90 days vs. WCD use >90 days enrolled in the WEARIT-II registry, further assessed by disease aetiology (ischaemic vs. non-ischaemic vs. congenital/inherited heart disease). There were 981 (49%) patients with WCD use >90 days, and 1019 patients with WCD use ≤90 days (median 120 vs. 55 days). There was a lower incidence of sustained ventricular tachycardia/ventricular fibrillation (VT/VF) events (11 vs. 50 events per 100 patient-years, P < 0.001), WCD treated VT/VF events (1 vs. 8 events per 100 patient-years, P < 0.001), and non-sustained VT events (21 vs. 51 events per 100 patient-years, P = 0.008) with WCD use >90 vs. WCD use ≤90 days. Non-ischaemic cardiomyopathy patients presented with similar rates of sustained VT/VF events during WCD use >90 vs. ≤90 days (13.4 vs. 13.7 events per 100 patient-years, P = 0.314), while most of these events terminated spontaneously. One-third of the patients with extended WCD use further improved their EF and they were not implanted with an ICD, with similar rates among ischaemic and non-ischaemic patients.
Conclusions: In WEARIT-II, patients with extended WCD use >90 days remain at risk for ventricular arrhythmia events. One-third of the patients with WCD use >90 days further improved their EF, avoiding the need to consider ICD implantation.
Figures
Comment in
-
The time is not ripe for the wearable cardioverter-defibrillator.Europace. 2018 Sep 1;20(FI2):f146-f147. doi: 10.1093/europace/euy135. Europace. 2018. PMID: 29905810 No abstract available.
-
Wearable cardioverter-defibrillator and ventricular arrhythmias: risk stratification in patients with shorter device use.Europace. 2019 Mar 1;21(3):525. doi: 10.1093/europace/euy283. Europace. 2019. PMID: 30561617 No abstract available.
-
Wearable cardioverter-defibrillator and ventricular arrhythmias: risk stratification in patients with shorter device use-Authors' reply.Europace. 2019 Mar 1;21(3):525-526. doi: 10.1093/europace/euy287. Europace. 2019. PMID: 30561623 No abstract available.
References
-
- Chung MK. The role of the wearable cardioverter defibrillator in clinical practice. Cardiol Clin 2014;32:253–70. - PubMed
-
- Piccini JP Sr, Allen LA, Kudenchuk PJ, Page RL, Patel MR, Turakhia MP.. Wearable cardioverter-defibrillator therapy for the prevention of sudden cardiac death: a science advisory from the American Heart Association. Circulation 2016;133:1715–27. - PubMed
-
- Reek S, Burri H, Roberts PR, Perings C, Epstein AE, Klein HU. et al. The wearable cardioverter-defibrillator: current technology and evolving indications. Europace 2017;19:335–45. - PubMed
-
- Feldman AM, Klein H, Tchou P, Murali S, Hall WJ, Mancini D. et al. Use of a wearable defibrillator in terminating tachyarrhythmias in patients at high risk for sudden death: results of the WEARIT/BIROAD. Pacing Clin Electrophysiol 2004;27:4–9. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

