Yeast as a tool to identify anti-aging compounds
- PMID: 29905792
- PMCID: PMC6001894
- DOI: 10.1093/femsyr/foy020
Yeast as a tool to identify anti-aging compounds
Abstract
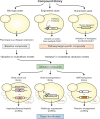
In the search for interventions against aging and age-related diseases, biological screening platforms are indispensable tools to identify anti-aging compounds among large substance libraries. The budding yeast, Saccharomyces cerevisiae, has emerged as a powerful chemical and genetic screening platform, as it combines a rapid workflow with experimental amenability and the availability of a wide range of genetic mutant libraries. Given the amount of conserved genes and aging mechanisms between yeast and human, testing candidate anti-aging substances in yeast gene-deletion or overexpression collections, or de novo derived mutants, has proven highly successful in finding potential molecular targets. Yeast-based studies, for example, have led to the discovery of the polyphenol resveratrol and the natural polyamine spermidine as potential anti-aging agents. Here, we present strategies for pharmacological anti-aging screens in yeast, discuss common pitfalls and summarize studies that have used yeast for drug discovery and target identification.
Figures



References
-
- Almeida B, Silva A, Mesquita A et al. Drug-induced apoptosis in yeast. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 2008;1783:1436–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

