Synthetic switch-based baculovirus for transgene expression control and selective killing of hepatocellular carcinoma cells
- PMID: 29905834
- PMCID: PMC6125686
- DOI: 10.1093/nar/gky447
Synthetic switch-based baculovirus for transgene expression control and selective killing of hepatocellular carcinoma cells
Abstract
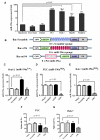
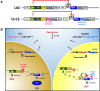
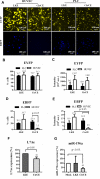
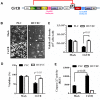
Baculovirus (BV) holds promise as a vector for anticancer gene delivery to combat the most common liver cancer-hepatocellular carcinoma (HCC). However, in vivo BV administration inevitably results in BV entry into non-HCC normal cells, leaky anticancer gene expression and possible toxicity. To improve the safety, we employed synthetic biology to engineer BV for transgene expression regulation. We first uncovered that miR-196a and miR-126 are exclusively expressed in HCC and normal cells, respectively, which allowed us to engineer a sensor based on distinct miRNA expression signature. We next assembled a synthetic switch by coupling the miRNA sensor and RNA binding protein L7Ae for translational repression, and incorporated the entire device into a single BV. The recombinant BV efficiently entered HCC and normal cells and enabled cis-acting transgene expression control, by turning OFF transgene expression in normal cells while switching ON transgene expression in HCC cells. Using pro-apoptotic hBax as the transgene, the switch-based BV selectively killed HCC cells in separate culture and mixed culture of HCC and normal cells. These data demonstrate the potential of synthetic switch-based BV to distinguish HCC and non-HCC normal cells for selective transgene expression control and killing of HCC cells.
Figures

References
-
- Delaney W.E., Miller T.G., Isom H.C.. Use of the hepatitis B virus recombinant baculovirus-HepG2 system to study the effects of (-)-beta-2′,3′-dideoxy-3′-thiacytidine on replication of hepatitis B virus and accumulation of covalently closed circular DNA. Antimicrob. Agents Chemother. 1999; 43:2017–2026. - PMC - PubMed
-
- Lin C.-Y., Lin K.-J., Kao C.-Y., Chen M.-C., Yen T.-Z., Lo W.-H., Chang Y.-H., Hu Y.-C.. The role of adipose-derived stem cells engineered with the persistently expressing hybrid baculovirus in the healing of massive bone defects. Biomaterials. 2011; 32:6505–6514. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

