Robotic Assisted MRI-Guided Interventional Interstitial MR-Guided Focused Ultrasound Ablation in a Swine Model
- PMID: 29905844
- PMCID: PMC6500887
- DOI: 10.1093/neuros/nyy266
Robotic Assisted MRI-Guided Interventional Interstitial MR-Guided Focused Ultrasound Ablation in a Swine Model
Abstract
Background: Ablative lesions are current treatments for epilepsy and brain tumors. Interstitial magnetic resonance (MR) guided focused ultrasound (iMRgFUS) may be an alternate ablation technique which limits thermal tissue charring as compared to laser therapy (LITT) and can produce larger ablation patterns nearer the surface than transcranial MR guided focused ultrasound (tcMRgFUS).
Objective: To describe our experience with interstitial focused ultrasound (iFUS) ablations in swine, using MR-guided robotically assisted (MRgRA) delivery.
Methods: In an initial 3 animals, we optimized the workflow of the robot in the MR suite and made modifications to the robotic arm to allow range of motion. Then, 6 farm pigs (4 acute, 2 survival) underwent 7 iMRgFUS ablations using MRgRA. We altered dosing to explore differences between thermal dosing in brain as compared to other tissues. Imaging was compared to gross examination.
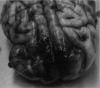
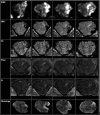
Results: Our work culminated in adjustments to the MRgRA, iMRgFUS probes, and dosing, culminating in 2 survival surgeries; swine had ablations with no neurological sequelae at 2 wk postprocedure. Immediately following iMRgFUS therapy, diffusion-weighted imaging, and T1 weighted MR were accurate reflections of the ablation volume. T2 and fluid-attenuated inversion-recovery (FLAIR) images were accurate reflections of ablation volume 1-wk postprocedure.
Conclusion: We successfully performed MRgRA iFUS ablation in swine and found intraoperative and postoperative imaging to correlate with histological examination. These data are useful to validate our system and to guide imaging follow-up for thermal ablation lesions in brain tissue from our therapy, tcMRgFUS, and LITT.
Keywords: Brain tumor; High intensity focused ultrasound; Interstitial focused ultrasound; MRI-Guided; Neural ablation; Robot assisted surgery.
Copyright © 2018 by the Congress of Neurological Surgeons.
Figures






References
-
- Missios S, Bekelis K, Barnett GH. Renaissance of laser interstitial thermal ablation. Neurosurg Focus. 2015;38(3):E13. - PubMed
-
- Kangasniemi M, Diederich CJ, Price RE et al.. Multiplanar MR temperature-sensitive imaging of cerebral thermal treatment using interstitial ultrasound applicators in a canine model. J Magn Reson Imaging. 2002;16(5):522–531. - PubMed
-
- Canney MS, Chavrier F, Tsysar S, Chapelon JY, Lafon C, Carpentier A. A multi-element interstitial ultrasound applicator for the thermal therapy of brain tumors. J Acoust Soc Am. 2013;134(2):1647–1655. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

