Hexafluoroisopropanol as the Acid Component in the Passerini Reaction: One-Pot Access to β-Amino Alcohols
- PMID: 29906122
- PMCID: PMC6038100
- DOI: 10.1021/acs.orglett.8b01561
Hexafluoroisopropanol as the Acid Component in the Passerini Reaction: One-Pot Access to β-Amino Alcohols
Abstract
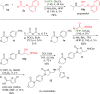
A new Passerini-type reaction in which hexafluoroisopropanol functions as the acid component is reported. The reaction tolerates a broad range of isocyanides and aldehydes, and the formed imidates can be reduced toward β-amino alcohols under mild and metal-free conditions. In addition, the imidate products were shown to undergo an unprecedented retro-Passerini-type reaction under microwave conditions, providing valuable mechanistic information about the Passerini reaction and its variations.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Bienaymé H.; Hulme C.; Oddon G.; Schmitt P. Chem. - Eur. J. 2000, 6, 3321. 10.1002/1521-3765(20000915)6:18<3321::AID-CHEM3321>3.0.CO;2-A. - DOI - PubMed
- Sunderhaus J. D.; Martin S. F. Chem. - Eur. J. 2009, 15, 1300. 10.1002/chem.200802140. - DOI - PMC - PubMed
- Dömling A.; Wang W.; Wang K. Chem. Rev. 2012, 112, 3083. 10.1021/cr100233r. - DOI - PMC - PubMed
- van der Heijden G.; Ruijter E.; Orru R. Synlett 2013, 24, 666. 10.1055/s-0032-1318222. - DOI
- Cioc R. C.; Ruijter E.; Orru R. V. A. Green Chem. 2014, 16, 2958. 10.1039/C4GC00013G. - DOI
- Rotstein B. H.; Zaretsky S.; Rai V.; Yudin A. K. Chem. Rev. 2014, 114, 8323. 10.1021/cr400615v. - DOI - PubMed
- Zarganes-Tzitzikas T.; Chandgude A. L.; Dömling A. Chem. Rec. 2015, 15, 981. 10.1002/tcr.201500201. - DOI - PubMed
-
- Dömling A.; Ugi I. Angew. Chem., Int. Ed. 2000, 39, 3168. 10.1002/1521-3773(20000915)39:18<3168::AID-ANIE3168>3.0.CO;2-U. - DOI - PubMed
- Akritopoulou-Zanze I. Curr. Opin. Chem. Biol. 2008, 12, 324. 10.1016/j.cbpa.2008.02.004. - DOI - PubMed
- van Berkel S. S.; Bögels B. G. M.; Wijdeven M. A.; Westermann B.; Rutjes F. P. J. T. Eur. J. Org. Chem. 2012, 2012, 3543. 10.1002/ejoc.201200030. - DOI
- Sadjadi S.; Heravi M. M.; Nazari N. RSC Adv. 2016, 6, 53203. 10.1039/C6RA02143C. - DOI
- Giustiniano M.; Basso A.; Mercalli V.; Massarotti A.; Novellino E.; Tron G. C.; Zhu J. Chem. Soc. Rev. 2017, 46, 1295. 10.1039/C6CS00444J. - DOI - PubMed
-
- Passerini M.; Simone L. Gazz. Chim. Ital. 1921, 51, 126.
-
- Ugi I.; Steinbrückner C. Chem. Ber. 1961, 94, 734. 10.1002/cber.19610940323. - DOI
-
- Tron G. C. Eur. J. Org. Chem. 2013, 2013, 1849. 10.1002/ejoc.201201660. - DOI
- El Kaïm L.; Grimaud L. Eur. J. Org. Chem. 2014, 2014, 7749. 10.1002/ejoc.201402783. - DOI
- Koopmanschap G.; Ruijter E.; Orru R. V. A. Beilstein J. Org. Chem. 2014, 10, 544. 10.3762/bjoc.10.50. - DOI - PMC - PubMed
- Maleki A.; Sarvary A. RSC Adv. 2015, 5, 60938. 10.1039/C5RA11531K. - DOI
- Sharma U. K.; Sharma N.; Vachhani D. D.; Van Der Eycken E. V. Chem. Soc. Rev. 2015, 44, 1836. 10.1039/C4CS00253A. - DOI - PubMed
- Shaaban S.; Abdel-Wahab B. F.; Saad Shaaban B. Mol. Diversity 2016, 20, 233. 10.1007/s11030-015-9602-6. - DOI - PubMed
- Banfi L.; Basso A.; Lambruschini C.; Moni L.; Riva R. Chem. Heterocycl. Compd. 2017, 53, 382. 10.1007/s10593-017-2065-1. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

