De novo lipogenesis represents a therapeutic target in mutant Kras non-small cell lung cancer
- PMID: 29906244
- PMCID: PMC6219836
- DOI: 10.1096/fj.201800204
De novo lipogenesis represents a therapeutic target in mutant Kras non-small cell lung cancer
Abstract
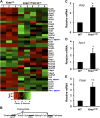
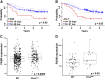
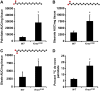
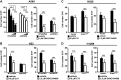
Oncogenic Kras mutations are one of the most common alterations in non-small cell lung cancer and are associated with poor response to treatment and reduced survival. Driver oncogenes, such as Kras are now appreciated for their ability to promote tumor growth via up-regulation of anabolic pathways. Therefore, we wanted to identify metabolic vulnerabilities in Kras-mutant lung cancer. Using the Kras LSL-G12D lung cancer model, we show that mutant Kras drives a lipogenic gene-expression program. Stable-isotope analysis reveals that mutant Kras promotes de novo fatty acid synthesis in vitro and in vivo. The importance of fatty acid synthesis in Kras-induced tumorigenesis was evident by decreased tumor formation in Kras LSL-G12D mice after treatment with a fatty acid synthesis inhibitor. Importantly, with gain and loss of function models of mutant Kras, we demonstrate that mutant Kras potentiates the growth inhibitory effects of several fatty acid synthesis inhibitors. These studies highlight the potential to target mutant Kras tumors by taking advantage of the lipogenic phenotype induced by mutant Kras.-Singh, A., Ruiz, C., Bhalla, K., Haley, J. A., Li, Q. K., Acquaah-Mensah, G., Montal, E., Sudini, K. R., Skoulidis, F., Wistuba, I. I., Papadimitrakopoulou, V., Heymach, J. V., Boros, L. G., Gabrielson, E., Carretero, J., Wong, K.-k., Haley, J. D., Biswal, S., Girnun, G. D. De novo lipogenesis represents a therapeutic target in mutant Kras non-small cell lung cancer.
Keywords: NSCLC; Warburg Effect; cancer metabolism.
Conflict of interest statement
The authors thank Prabhanshu Tripathi (Bloomberg School of Public Health, Johns Hopkins University) for help with the mouse studies and Ellen Tully (Johns Hopkins University) for assistance with the immunohistochemical studies. This work was supported by U.S. National Institutes of Health, National Cancer Institute Grants CA169919 (to G.D.G.) and R01CA206155 (to S.B.); a research grant from the Maryland Cigarette Restitution Fund (to S.B., G.G., and A.S.); and a Young Clinical Innovator Award (to A.S.) from the Flight Attendant Medical Research Institute. We apologize to those laboratories that we were not able to reference. The authors declare no conflicts of interest.
Figures

References
-
- Aviel-Ronen S., Blackhall F. H., Shepherd F. A., Tsao M. S. (2006) K-ras mutations in non-small-cell lung carcinoma: a review. Clin. Lung Cancer 8, 30–38 - PubMed
-
- Bos J. L. (1989) ras oncogenes in human cancer: a review. Cancer Res. 49, 4682–4689 - PubMed
-
- Huncharek M., Muscat J., Geschwind J. F. (1999) K-ras oncogene mutation as a prognostic marker in non-small cell lung cancer: a combined analysis of 881 cases. Carcinogenesis 20, 1507–1510 - PubMed
-
- Mascaux C., Iannino N., Martin B., Paesmans M., Berghmans T., Dusart M., Haller A., Lothaire P., Meert A. P., Noel S., Lafitte J. J., Sculier J. P. (2005) The role of RAS oncogene in survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br. J. Cancer 92, 131–139 - PMC - PubMed
-
- Matikas A., Mistriotis D., Georgoulias V., Kotsakis A. (2017) Targeting KRAS mutated non-small cell lung cancer: a history of failures and a future of hope for a diverse entity. Crit. Rev. Oncol. Hematol. 110, 1–12 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

