Genomic Dissection of Bipolar Disorder and Schizophrenia, Including 28 Subphenotypes
- PMID: 29906448
- PMCID: PMC6432650
- DOI: 10.1016/j.cell.2018.05.046
Genomic Dissection of Bipolar Disorder and Schizophrenia, Including 28 Subphenotypes
Abstract
Schizophrenia and bipolar disorder are two distinct diagnoses that share symptomology. Understanding the genetic factors contributing to the shared and disorder-specific symptoms will be crucial for improving diagnosis and treatment. In genetic data consisting of 53,555 cases (20,129 bipolar disorder [BD], 33,426 schizophrenia [SCZ]) and 54,065 controls, we identified 114 genome-wide significant loci implicating synaptic and neuronal pathways shared between disorders. Comparing SCZ to BD (23,585 SCZ, 15,270 BD) identified four genomic regions including one with disorder-independent causal variants and potassium ion response genes as contributing to differences in biology between the disorders. Polygenic risk score (PRS) analyses identified several significant correlations within case-only phenotypes including SCZ PRS with psychotic features and age of onset in BD. For the first time, we discover specific loci that distinguish between BD and SCZ and identify polygenic components underlying multiple symptom dimensions. These results point to the utility of genetics to inform symptomology and potential treatment.
Keywords: bipolar disorder; polygenic risk; psychosis; schizophrenia; subphenotypes.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests
The authors declare no competing interests.
Figures
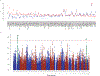
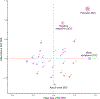
References
-
- Amare AT, Schubert KO, Hou L, Clark SR, Papiol S, Heilbronner U, Degenhardt F, Tekola-Ayele F, Hsu Y-H, Shekhtman T, Adli M, Akula N, Akiyama K, Ardau R, Arias B, Aubry J-M, Backlund L, Bhattacharjee AK, Bellivier F, Benabarre A, Bengesser S, Biernacka JM, Birner A, Brichant-Petitjean C, Cervantes P, Chen H-C, Chillotti C, Cichon S, Cruceanu C, Czerski PM, Dalkner N, Dayer A, Zompo MD, DePaulo JR, Étain B, Falkai P, Forstner AJ, Frisen L, Frye MA, Fullerton JM, Gard S, Garnham JS, Goes FS, Grigoroiu-Serbanescu M, Grof P, Hashimoto R, Hauser J, Herms S, Hoffmann P, Hofmann A, Jamain S, Jiménez E, Kahn J-P, Kassem L, Kuo P-H, Kato T, Kelsoe J, Kittel-Schneider S, Kliwicki S, König B, Kusumi I, Laje G, Landén M, Lavebratt C, Leboyer M, Leckband SG, Tortorella A, Manchia M, Martinsson L, McCarthy MJ, McElroy S, Colom F, Mitjans M, Mondimore FM, Monteleone P, Nievergelt CM, Nöthen MM, Novák T, O’Donovan C, Ozaki N, Ösby U, Pfennig A, Potash JB, Reif A, Reininghaus E, Rouleau GA, Rybakowski JK, Schalling M, Schofield PR, Schweizer BW, Severino G, Shilling PD, Shimoda K, Simhandl C, Slaney CM, Squassina A, Stamm T, Stopkova P, Maj M, Turecki G, Vieta E, Volkert J, Witt S, Wright A, Zandi PP, Mitchell PB, Bauer M, Alda M, Rietschel M, McMahon FJ, Schulze TG, Baune BT, 2018. Association of Polygenic Score for Schizophrenia and HLA Antigen and Inflammation Genes With Response to Lithium in Bipolar Affective Disorder: A Genome-Wide Association Study. JAMA Psychiatry 75, 65–74. 10.1001/jamapsychiatry.2017.3433 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- R01 MH104964/MH/NIMH NIH HHS/United States
- G1000708/MRC_/Medical Research Council/United Kingdom
- MR/M008436/1/MRC_/Medical Research Council/United Kingdom
- MR/P005748/1/MRC_/Medical Research Council/United Kingdom
- U01 MH109536/MH/NIMH NIH HHS/United States
- U01 MH109514/MH/NIMH NIH HHS/United States
- R01 MH085548/MH/NIMH NIH HHS/United States
- R00 MH101367/MH/NIMH NIH HHS/United States
- R01 MH111776/MH/NIMH NIH HHS/United States
- R01 MH123451/MH/NIMH NIH HHS/United States
- MR/L023784/2/MRC_/Medical Research Council/United Kingdom
- MR/L010305/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

