Changes in biomarkers in equine synovial fluid two weeks after intra-articular hyaluronan treatment: a randomised double-blind clinical trial
- PMID: 29907111
- PMCID: PMC6003042
- DOI: 10.1186/s12917-018-1512-2
Changes in biomarkers in equine synovial fluid two weeks after intra-articular hyaluronan treatment: a randomised double-blind clinical trial
Abstract
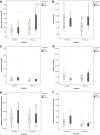
Background: Inflammatory and degenerative activity inside the joint can be studied in vivo via analysis of synovial fluid (SF) biomarkers, which are molecular markers of inflammatory processes and tissue turnover. The aim of this study was to investigate the response of selected biomarkers in the SF after an intra-articular (IA) high-molecular-weight non-animal stabilized hyaluronic acid (NASHA) treatment. Our hypothesis was that prostaglandin E2 (PGE2), substance P, aggrecan chondroitin sulfate 846 epitope (CS846), and carboxypeptide of type II collagen (CPII) concentrations in SF would decrease more in the NASHA than in the placebo group. Twenty-eight clinically lame horses with positive responses to diagnostic IA anaesthesia of the metacarpophalangeal or metatarsophalangeal joints were randomized into treatment (n = 15) and control (n = 13) groups. After collection of baseline SF samples followed by IA diagnostic anaesthesia, horses in the treatment group received 3 ml of a NASHA product IA. Those in the placebo group received an equivalent volume of sterile 0.9% saline solution. The horses were re-evaluated and a second SF sample was obtained after a 2-week period.
Results: CS846 concentration decreased in the NASHA group only (P = 0.010). Both PGE2 and CPII concentrations decreased within the groups (PGE2, P = 0.010 for the NASHA group; P = 0.027 for the placebo group; CPII, P < 0.001 for NASHA group; P = 0.009 for placebo group). No significant treatment effect for any biomarker was found between groups. NASHA induced an increase in white blood cell count; this was significant compared with baseline (P = 0.021) and the placebo group (P = 0.045).
Conclusions: Although the SF concentration of the cartilage-derived biomarker CS846 decreased in the NASHA group, no statistically significant treatment effect of any of the biomarkers were observed between treatment groups. The significant increase in SF white blood cell count after IA NASHA may indicate a mild inflammatory response. However, as no clinical adverse effects were observed, we conclude that IA NASHA was well tolerated.
Keywords: Aggrecan chondroitin sulfate 846 epitope; Biomarkers; Carboxypeptide of type II collagen; Clinical study; Non-animal stabilized hyaluronic acid (NASHA); Placebo-controlled; Prostaglandin E2; Substance P.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved by the Viikki Campus Research Ethics Committee of the University of Helsinki. All horse owners signed a study consent before the start of the study and owners were allowed withdraw from the study without giving any particular reason.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Ruth DT, Swites BJ. Comparison of the effectiveness of intra-articular hyaluronic acid and conventional therapy for the treatment of naturally occurring arthritic conditions in horses. Equine Pract. 1985;7:25–29.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

