NF-κB signaling and its relevance to the treatment of mantle cell lymphoma
- PMID: 29907126
- PMCID: PMC6002979
- DOI: 10.1186/s13045-018-0621-5
NF-κB signaling and its relevance to the treatment of mantle cell lymphoma
Abstract
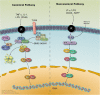
Mantle cell lymphoma is an aggressive subtype of non-Hodgkin B cell lymphoma that is characterized by a poor prognosis determined by Ki67 and Mantle Cell International Prognostic Index scores, but it is becoming increasingly treatable. The majority of patients, especially if young, achieve a progression-free survival of at least 5 years. Mantle cell lymphoma can initially be treated with an anti-CD20 antibody in combination with a chemotherapy backbone, such as VR-CAP (the anti-CD20 monoclonal antibody rituximab administered with cyclophosphamide, doxorubicin, and prednisone) or R-CHOP (the anti-CD20 monoclonal antibody rituximab administered with cyclophosphamide, doxorubicin, vincristine, and prednisone). While initial treatment can facilitate recovery and complete remission in a few patients, many patients experience relapsed or refractory mantle cell lymphoma within 2 to 3 years after initial treatment. Targeted agents such as ibrutinib, an inhibitor of Bruton's tyrosine kinase, which has been approved only in the relapsed setting, can be used to treat patients with relapsed or refractory mantle cell lymphoma. However, mantle cell lymphoma cells often acquire resistance to such targeted agents and continue to survive by activating alternate signaling pathways such as the PI3K-Akt pathway or the NF-κB pathways.NF-κB is a transcription factor family that regulates the growth and survival of B cells; mantle cell lymphoma cells depend on NF-κB signaling for continued growth and proliferation. The NF-κB signaling pathways are categorized into canonical and non-canonical types, wherein the canonical pathway prompts inflammatory responses, immune regulation, and cell proliferation, while the non-canonical leads to B cell maturation and lymphoid organogenesis. Since these pathways upregulate survival genes and tumor-promoting cytokines, they can be activated to overcome the inhibitory effects of targeted agents, thereby having profound effects on tumorigenesis. The NF-κB pathways are also highly targetable in that they are interconnected with numerous other pathways, including B cell receptor signaling, PI3K/Akt/mTOR signaling, and toll-like receptor signaling pathways. Additionally, elements of the non-canonical NF- κB pathway, such as NF-κB-inducing kinase, can be targeted to overcome resistance to targeting of the canonical NF- κB pathway.Targeting the molecular mechanisms of the NF-κB pathways can facilitate the development of novel agents to treat malignancies and overcome drug resistance in patients with relapsed or refractory mantle cell lymphoma.
Keywords: Canonical pathway; Mantle cell lymphoma; NF-κB; Non-canonical pathway.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Broide DH, Lawrence T, Doherty T, Cho JY, Miller M, McElwain K, McElwain S, Karin M. Allergen-induced peribronchial fibrosis and mucus production mediated by IkappaB kinase beta-dependent genes in airway epithelium. Proc Natl Acad Sci U S A. 2005;102:17723–17728. doi: 10.1073/pnas.0509235102. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

