Emerging role of lipid metabolism alterations in Cancer stem cells
- PMID: 29907133
- PMCID: PMC6003041
- DOI: 10.1186/s13046-018-0784-5
Emerging role of lipid metabolism alterations in Cancer stem cells
Erratum in
-
Correction to: Emerging role of lipid metabolism alterations in Cancer stem cells.J Exp Clin Cancer Res. 2018 Jul 16;37(1):155. doi: 10.1186/s13046-018-0826-z. J Exp Clin Cancer Res. 2018. PMID: 30012174 Free PMC article.
Abstract
Background: Cancer stem cells (CSCs) or tumor-initiating cells (TICs) represent a small population of cancer cells with self-renewal and tumor-initiating properties. Unlike the bulk of tumor cells, CSCs or TICs are refractory to traditional therapy and are responsible for relapse or disease recurrence in cancer patients. Stem cells have distinct metabolic properties compared to differentiated cells, and metabolic rewiring contributes to self-renewal and stemness maintenance in CSCs.
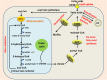
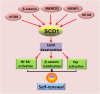
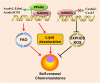
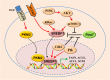
Main body: Recent advances in metabolomic detection, particularly in hyperspectral-stimulated raman scattering microscopy, have expanded our knowledge of the contribution of lipid metabolism to the generation and maintenance of CSCs. Alterations in lipid uptake, de novo lipogenesis, lipid droplets, lipid desaturation, and fatty acid oxidation are all clearly implicated in CSCs regulation. Alterations on lipid metabolism not only satisfies the energy demands and biomass production of CSCs, but also contributes to the activation of several important oncogenic signaling pathways, including Wnt/β-catenin and Hippo/YAP signaling. In this review, we summarize the current progress in this attractive field and describe some recent therapeutic agents specifically targeting CSCs based on their modulation of lipid metabolism.
Conclusion: Increased reliance on lipid metabolism makes it a promising therapeutic strategy to eliminate CSCs. Targeting key players of fatty acids metabolism shows promising to anti-CSCs and tumor prevention effects.
Keywords: Cancer stem cells; Fatty acid oxidation; Lipid desaturation; Lipid droplets; Lipid metabolism; Metabolomics; de novo lipogenesis.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable
Consent for publication
All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Targeting autophagy and lipid metabolism in cancer stem cells.Biochem Pharmacol. 2023 Jun;212:115550. doi: 10.1016/j.bcp.2023.115550. Epub 2023 Apr 13. Biochem Pharmacol. 2023. PMID: 37060962 Review.
-
Aberrant lipid metabolism as an emerging therapeutic strategy to target cancer stem cells.Stem Cells. 2020 Jan;38(1):6-14. doi: 10.1002/stem.3101. Epub 2019 Oct 31. Stem Cells. 2020. PMID: 31648395 Review.
-
Lipid metabolism alteration contributes to and maintains the properties of cancer stem cells.Theranostics. 2020 May 30;10(16):7053-7069. doi: 10.7150/thno.41388. eCollection 2020. Theranostics. 2020. PMID: 32641978 Free PMC article. Review.
-
Targeting Lipid Metabolism in Cancer Stem Cells for Anticancer Treatment.Int J Mol Sci. 2024 Oct 17;25(20):11185. doi: 10.3390/ijms252011185. Int J Mol Sci. 2024. PMID: 39456967 Free PMC article. Review.
-
Targeting autophagy in cancer stem cells as an anticancer therapy.Cancer Lett. 2017 May 1;393:33-39. doi: 10.1016/j.canlet.2017.02.012. Epub 2017 Feb 17. Cancer Lett. 2017. PMID: 28216370 Review.
Cited by
-
Discovery of a novel lipid metabolism-related gene signature to predict outcomes and the tumor immune microenvironment in gastric cancer by integrated analysis of single-cell and bulk RNA sequencing.Lipids Health Dis. 2023 Dec 2;22(1):212. doi: 10.1186/s12944-023-01977-y. Lipids Health Dis. 2023. PMID: 38042786 Free PMC article.
-
Screening of Lipid Metabolism-Related Genes as Diagnostic Indicators in Chronic Obstructive Pulmonary Disease.Int J Chron Obstruct Pulmon Dis. 2023 Nov 28;18:2739-2754. doi: 10.2147/COPD.S428984. eCollection 2023. Int J Chron Obstruct Pulmon Dis. 2023. PMID: 38046983 Free PMC article.
-
Serum untargeted lipidomics by UHPLC-ESI-HRMS aids the biomarker discovery of colorectal adenoma.BMC Cancer. 2022 Mar 24;22(1):314. doi: 10.1186/s12885-022-09427-1. BMC Cancer. 2022. PMID: 35331175 Free PMC article.
-
Correction to: Emerging role of lipid metabolism alterations in Cancer stem cells.J Exp Clin Cancer Res. 2018 Jul 16;37(1):155. doi: 10.1186/s13046-018-0826-z. J Exp Clin Cancer Res. 2018. PMID: 30012174 Free PMC article.
-
Parabacteroides distasonis uses dietary inulin to suppress NASH via its metabolite pentadecanoic acid.Nat Microbiol. 2023 Aug;8(8):1534-1548. doi: 10.1038/s41564-023-01418-7. Epub 2023 Jun 29. Nat Microbiol. 2023. PMID: 37386075 Free PMC article.
References
-
- Guen VJ, Chavarria TE, Kroger C, Ye X, Weinberg RA, Lees JA. EMT programs promote basal mammary stem cell and tumor-initiating cell stemness by inducing primary ciliogenesis and hedgehog signaling. Proc Natl Acad Sci U S A. 2017;114(49):E10532–E10539. doi: 10.1073/pnas.1711534114. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

