Doxorubicin-induced cardiotoxicity is suppressed by estrous-staged treatment and exogenous 17β-estradiol in female tumor-bearing spontaneously hypertensive rats
- PMID: 29907135
- PMCID: PMC6003183
- DOI: 10.1186/s13293-018-0183-9
Doxorubicin-induced cardiotoxicity is suppressed by estrous-staged treatment and exogenous 17β-estradiol in female tumor-bearing spontaneously hypertensive rats
Abstract
Background: Doxorubicin (DOX), an anthracycline therapeutic, is widely used to treat a variety of cancer types and known to induce cardiomyopathy in a time and dose-dependent manner. Postmenopausal and hypertensive females are two high-risk groups for developing adverse effects following DOX treatment. This may suggest that endogenous reproductive hormones can in part suppress DOX-induced cardiotoxicity. Here, we investigated if the endogenous fluctuations in 17β-estradiol (E2) and progesterone (P4) can in part suppress DOX-induced cardiomyopathy in SST-2 tumor-bearing spontaneously hypersensitive rats (SHRs) and evaluate if exogenous administration of E2 and P4 can suppress DOX-induced cardiotoxicity in tumor-bearing ovariectomized SHRs (ovaSHRs).
Methods: Vaginal cytology was performed on all animals to identify the stage of the estrous cycle. Estrous-staged SHRs received a single injection of saline, DOX, dexrazoxane (DRZ), or DOX combined with DRZ. OvaSHRs were implanted with time-releasing pellets that contained a carrier matrix (control), E2, P4, Tamoxifen (Tam), and combinations of E2 with P4 and Tam. Hormone pellet-implanted ovaSHRs received a single injection of saline or DOX. Cardiac troponin I (cTnI), E2, and P4 serum concentrations were measured before and after treatment in all animals. Cardiac damage and function were further assessed by echocardiography and histopathology. Weight, tumor size, and uterine width were measured for all animals.
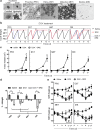
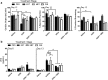
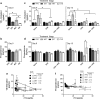
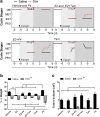
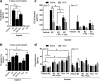
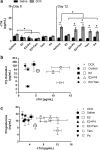
Results: In SHRs, estrous-staged DOX treatment altered acute estrous cycling that ultimately resulted in prolonged diestrus. Twelve days after DOX administration, all SHRs had comparable endogenous circulating E2. Thirteen days after DOX treatment, SHRs treated during proestrus had decreased cardiac output and increased cTnI as compared to animals treated during estrus and diestrus. DOX-induced tumor reduction was not affected by estrous-staged treatments. In ovaSHRs, exogenous administration of E2 suppressed DOX-induced cardiotoxicity, while P4-implanted ovaSHRs were partly resistant. However, ovaSHRs treated with E2 and P4 did not have cardioprotection against DOX-induced damage.
Conclusions: This study demonstrates that estrous-staged treatments can alter the extent of cardiac damage caused by DOX in female SHRs. The study also supports that exogenous E2 can suppress DOX-induced myocardial damage in ovaSHRs.
Keywords: Adriamycin; Cardiomyopathy; Cardioprotection; Doxorubicin; Estradiol; Progesterone.
Conflict of interest statement
Authors’ disclosures
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the United States Food and Drug Administration and the Department of Health and Human Services, nor does the mention of trade names, commercial products, or organizations imply endorsement by the United States Government.
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Reproductive hormone levels and differential mitochondria-related oxidative gene expression as potential mechanisms for gender differences in cardiosensitivity to Doxorubicin in tumor-bearing spontaneously hypertensive rats.Cancer Chemother Pharmacol. 2015 Sep;76(3):447-59. doi: 10.1007/s00280-015-2786-8. Epub 2015 Jun 25. Cancer Chemother Pharmacol. 2015. PMID: 26108538
-
The effects of cycling levels of 17beta-estradiol and progesterone on the magnitude of temporomandibular joint-induced nociception.Endocrinology. 2009 Aug;150(8):3680-9. doi: 10.1210/en.2008-1707. Epub 2009 Apr 9. Endocrinology. 2009. PMID: 19359384 Free PMC article.
-
17β-estradiol protects against doxorubicin-induced cardiotoxicity in male Sprague-Dawley rats by regulating NADPH oxidase and apoptosis genes.Mol Med Rep. 2017 May;15(5):2695-2702. doi: 10.3892/mmr.2017.6332. Epub 2017 Mar 16. Mol Med Rep. 2017. PMID: 28447737
-
Molecular mechanisms of doxorubicin-induced cardiotoxicity: novel roles of sirtuin 1-mediated signaling pathways.Cell Mol Life Sci. 2021 Apr;78(7):3105-3125. doi: 10.1007/s00018-020-03729-y. Epub 2021 Jan 13. Cell Mol Life Sci. 2021. PMID: 33438055 Free PMC article. Review.
-
Pharmaceutical Measures to Prevent Doxorubicin-Induced Cardiotoxicity.Mini Rev Med Chem. 2017;17(1):44-50. doi: 10.2174/1389557516666160621083659. Mini Rev Med Chem. 2017. PMID: 27337969 Review.
Cited by
-
Sex-Specific Cardiovascular Risks of Cancer and Its Therapies.Circ Res. 2022 Feb 18;130(4):632-651. doi: 10.1161/CIRCRESAHA.121.319901. Epub 2022 Feb 17. Circ Res. 2022. PMID: 35175846 Free PMC article. Review.
-
Anthracycline chemotherapy-mediated vascular dysfunction as a model of accelerated vascular aging.Aging Cancer. 2021 Jun;2(1-2):45-69. doi: 10.1002/aac2.12033. Epub 2021 Jun 22. Aging Cancer. 2021. PMID: 34212156 Free PMC article.
-
p38δ genetic ablation protects female mice from anthracycline cardiotoxicity.Am J Physiol Heart Circ Physiol. 2020 Oct 1;319(4):H775-H786. doi: 10.1152/ajpheart.00415.2020. Epub 2020 Aug 21. Am J Physiol Heart Circ Physiol. 2020. PMID: 32822209 Free PMC article.
-
Sexual dimorphism of acute doxorubicin-induced nephrotoxicity in C57Bl/6 mice.PLoS One. 2019 Feb 20;14(2):e0212486. doi: 10.1371/journal.pone.0212486. eCollection 2019. PLoS One. 2019. PMID: 30785938 Free PMC article.
-
Cardioprotective effects of exercise training on doxorubicin-induced cardiomyopathy: a systematic review with meta-analysis of preclinical studies.Sci Rep. 2021 Mar 18;11(1):6330. doi: 10.1038/s41598-021-83877-8. Sci Rep. 2021. PMID: 33737561 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

