Cytokine release syndrome
- PMID: 29907163
- PMCID: PMC6003181
- DOI: 10.1186/s40425-018-0343-9
Cytokine release syndrome
Abstract
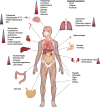
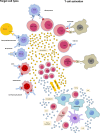
During the last decade the field of cancer immunotherapy has witnessed impressive progress. Highly effective immunotherapies such as immune checkpoint inhibition, and T-cell engaging therapies like bispecific T-cell engaging (BiTE) single-chain antibody constructs and chimeric antigen receptor (CAR) T cells have shown remarkable efficacy in clinical trials and some of these agents have already received regulatory approval. However, along with growing experience in the clinical application of these potent immunotherapeutic agents comes the increasing awareness of their inherent and potentially fatal adverse effects, most notably the cytokine release syndrome (CRS). This review provides a comprehensive overview of the mechanisms underlying CRS pathophysiology, risk factors, clinical presentation, differential diagnoses, and prognostic factors. In addition, based on the current evidence we give practical guidance to the management of the cytokine release syndrome.
Keywords: CAR T cells; Cytokine release syndrome; Cytokine storm; Immunotherapy; T cell-engaging therapies.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Chatenoud L, Ferran C, Reuter A, Legendre C, Gevaert Y, Kreis H, et al. Systemic reaction to the anti-T-cell monoclonal antibody OKT3 in relation to serum levels of tumor necrosis factor and interferon-gamma [corrected] N Engl J Med. 1989;320:1420–1421. doi: 10.1056/NEJM198905253202117. - DOI - PubMed
-
- Winkler U, Jensen M, Manzke O, Schulz H, Diehl V, Engert A. Cytokine-release syndrome in patients with B-cell chronic lymphocytic leukemia and high lymphocyte counts after treatment with an anti-CD20 monoclonal antibody (rituximab, IDEC-C2B8). Blood. 1999;94 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
