Diversity of rotavirus strains circulating in children under five years of age who presented with acute gastroenteritis before and after rotavirus vaccine introduction, University Teaching Hospital, Lusaka, Zambia, 2008-2015
- PMID: 29907481
- PMCID: PMC11973881
- DOI: 10.1016/j.vaccine.2018.03.035
Diversity of rotavirus strains circulating in children under five years of age who presented with acute gastroenteritis before and after rotavirus vaccine introduction, University Teaching Hospital, Lusaka, Zambia, 2008-2015
Abstract
Background: Following the introduction of rotavirus vaccine into the routine immunization schedule, the burden of rotavirus disease has significantly reduced in Zambia. Although rotavirus vaccines appear to confer good cross-protection against both vaccine and non-vaccine strains, concerns about strain replacement following vaccine implementation remain. We describe the diversity of the circulating rotavirus strains before and after the Rotarix® vaccine was introduced in Lusaka from January 2012.
Methods: Under five children were enrolled through active surveillance at University Teaching Hospital using a standardized WHO case investigation form. Stool samples were collected from children who presented with ≥3 loose stool in 24 h and were admitted to the hospital for acute gastroenteritis as a primary illness. Samples were tested for group A rotavirus antigen enzyme-linked immunosorbent assay. Randomly selected rotavirus positive samples were analysed by reverse transcription polymerase chain reaction for G and P genotyping and and Nucleotide sequencing was used to confirm some mixed infections.
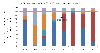
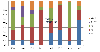
Results: A total of 4150 cases were enrolled and stool samples were collected from 4066 (98%) children between 2008 and 2011, before the vaccine was introduced. Rotavirus antigen was detected in 1561/4066 (38%). After vaccine introduction (2012 to 2015), 3168 cases were enrolled, 3092 (98%) samples were collected, and 977/3092 (32%) were positive for rotavirus. The most common G and P genotype combinations before vaccine introduction were G1P[8] (49%) in 2008; G12P[6] (24%) and G9P[8] (22%) in 2009; mixed rotavirus infections (32%) and G9P[8] (20%) in 2010, and G1P[6] (46%), G9P[6] (16%) and mixed infections (20%) in 2011. The predominant strains after vaccine introduction were G1P[8] (25%), G2P[4] (28%) and G2P[6] (23%) in 2012; G2P[4] (36%) and G2P[6] (44%) in 2013; G1P[8] (43%), G2P[4] (9%), and G2P[6] (24%) in 2014, while G2P[4] (54%) and G2P[6] (20%) continued to circulate in 2015.
Conclusion: These continual changes in the predominant strains suggest natural secular variation in circulating rotavirus strains post-vaccine introduction. These findings highlight the need for ongoing surveillance to continue monitoring how vaccine use affects strain evolution over a longer period of time and assess any normal seasonal fluctuations of the rotavirus strains.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures
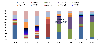
References
-
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, et al. (2012) 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 12: 136–141 - PubMed
-
- Matthijnssens J, Ciarlet M, McDonald SM, Attoui H, Bányai K, Brister JR, Buesa J, Esona MD, Estes MK, Gentsch JR, Iturriza-Gómara M. Uniformity of rotavirus strain nomenclature proposed by the Rotavirus Classification Working Group (RCWG). Archives of virology. 2011. Aug 1;156(8):1397–413.. - PMC - PubMed
-
- Abe M, Ito N, Masatani T, Nakagawa K, Yamaoka S, Kanamaru Y, et al. Whole genome characterization of new bovine rotavirus G21P [29] and G24P [33] strains provides evidence for interspecies transmission. J Gen Virol 2011. Apr 1;92(4):952–60. - PubMed
Further Reading
-
- . Justino MC, Linhares AC, Lanzieri TM, Miranda Y, Mascarenhas JD, Abreu E, Guerra SF, Oliveira AS, Da Silva VB, Sanchez N, Meyer N. Effectiveness of the monovalent G1P [8] human rotavirus vaccine against hospitalization for severe G2P [4] rotavirus gastroenteritis in Belem, Brazil. The Pediatric infectious disease journal. 2011. May 1;30(5):396–401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

