Beyond maximum grade: modernising the assessment and reporting of adverse events in haematological malignancies
- PMID: 29907552
- PMCID: PMC6261436
- DOI: 10.1016/S2352-3026(18)30051-6
Beyond maximum grade: modernising the assessment and reporting of adverse events in haematological malignancies
Erratum in
-
Correction to Lancet Haematol 2018; 5: e563-98.Lancet Haematol. 2019 Mar;6(3):e121. doi: 10.1016/S2352-3026(19)30016-X. Lancet Haematol. 2019. PMID: 30824039 No abstract available.
Abstract
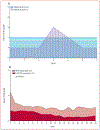
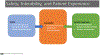
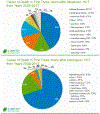
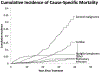
Tremendous progress in treatment and outcomes has been achieved across the whole range of haematological malignancies in the past two decades. Although cure rates for aggressive malignancies have increased, nowhere has progress been more impactful than in the management of typically incurable forms of haematological cancer. Population-based data have shown that 5-year survival for patients with chronic myelogenous and chronic lymphocytic leukaemia, indolent B-cell lymphomas, and multiple myeloma has improved markedly. This improvement is a result of substantial changes in disease management strategies in these malignancies. Several haematological malignancies are now chronic diseases that are treated with continuously administered therapies that have unique side-effects over time. In this Commission, an international panel of clinicians, clinical investigators, methodologists, regulators, and patient advocates representing a broad range of academic and clinical cancer expertise examine adverse events in haematological malignancies. The issues pertaining to assessment of adverse events examined here are relevant to a range of malignancies and have been, to date, underexplored in the context of haematology. The aim of this Commission is to improve toxicity assessment in clinical trials in haematological malignancies by critically examining the current process of adverse event assessment, highlighting the need to incorporate patient-reported outcomes, addressing issues unique to stem-cell transplantation and survivorship, appraising challenges in regulatory approval, and evaluating toxicity in real-world patients. We have identified a range of priority issues in these areas and defined potential solutions to challenges associated with adverse event assessment in the current treatment landscape of haematological malignancies.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures




Comment in
-
Eligibility criteria in haematological cancer clinical trials-what's needed?Lancet Haematol. 2020 Jan;7(1):e10. doi: 10.1016/S2352-3026(19)30160-7. Epub 2019 Dec 5. Lancet Haematol. 2020. PMID: 31813851 No abstract available.
References
-
- Stilgenbauer S, Eichhorst B, Schetelig J, Coutre S, Seymour JF, Munir T, et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study. Lancet Oncol. 2016;17(6):768–78. - PubMed
-
- Marcus R, Davies A, Ando K, Klapper W, Opat S, Owen C, et al. Obinutuzumab for the First-Line Treatment of Follicular Lymphoma. N Engl J Med. 2017;377(14):1331–44. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

