Structure of human ADP-ribosyl-acceptor hydrolase 3 bound to ADP-ribose reveals a conformational switch that enables specific substrate recognition
- PMID: 29907568
- PMCID: PMC6093245
- DOI: 10.1074/jbc.RA118.003586
Structure of human ADP-ribosyl-acceptor hydrolase 3 bound to ADP-ribose reveals a conformational switch that enables specific substrate recognition
Abstract
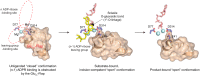
ADP-ribosyl-acceptor hydrolase 3 (ARH3) plays important roles in regulation of poly(ADP-ribosyl)ation, a reversible post-translational modification, and in maintenance of genomic integrity. ARH3 degrades poly(ADP-ribose) to protect cells from poly(ADP-ribose)-dependent cell death, reverses serine mono(ADP-ribosyl)ation, and hydrolyzes O-acetyl-ADP-ribose, a product of Sirtuin-catalyzed histone deacetylation. ARH3 preferentially hydrolyzes O-linkages attached to the anomeric C1″ of ADP-ribose; however, how ARH3 specifically recognizes and cleaves structurally diverse substrates remains unknown. Here, structures of full-length human ARH3 bound to ADP-ribose and Mg2+, coupled with computational modeling, reveal a dramatic conformational switch from closed to open states that enables specific substrate recognition. The glutamate flap, which blocks substrate entrance to Mg2+ in the unliganded closed state, is ejected from the active site when substrate is bound. This closed-to-open transition significantly widens the substrate-binding channel and precisely positions the scissile 1″-O-linkage for cleavage while securing tightly 2″- and 3″-hydroxyls of ADP-ribose. Our collective data uncover an unprecedented structural plasticity of ARH3 that supports its specificity for the 1″-O-linkage in substrates and Mg2+-dependent catalysis.
Keywords: ADP-ribosylation; ARH3; PARP1; conformational change; hydrolase; structural biology; substrate specificity.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Figures





References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

