NADPH Oxidase 5 Is a Pro-Contractile Nox Isoform and a Point of Cross-Talk for Calcium and Redox Signaling-Implications in Vascular Function
- PMID: 29907654
- PMCID: PMC6220544
- DOI: 10.1161/JAHA.118.009388
NADPH Oxidase 5 Is a Pro-Contractile Nox Isoform and a Point of Cross-Talk for Calcium and Redox Signaling-Implications in Vascular Function
Abstract
Background: NADPH Oxidase 5 (Nox5) is a calcium-sensitive superoxide-generating Nox. It is present in lower forms and higher mammals, but not in rodents. Nox5 is expressed in vascular cells, but the functional significance remains elusive. Given that contraction is controlled by calcium and reactive oxygen species, both associated with Nox5, we questioned the role of Nox5 in pro-contractile signaling and vascular function.
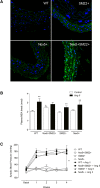
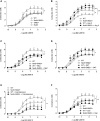
Methods and results: Transgenic mice expressing human Nox5 in a vascular smooth muscle cell-specific manner (Nox5 mice) and Rhodnius prolixus, an arthropod model that expresses Nox5 endogenoulsy, were studied. Reactive oxygen species generation was increased systemically and in the vasculature and heart in Nox5 mice. In Nox5-expressing mice, agonist-induced vasoconstriction was exaggerated and endothelium-dependent vasorelaxation was impaired. Vascular structural and mechanical properties were not influenced by Nox5. Vascular contractile responses in Nox5 mice were normalized by N-acetylcysteine and inhibitors of calcium channels, calmodulin, and endoplasmic reticulum ryanodine receptors, but not by GKT137831 (Nox1/4 inhibitor). At the cellular level, vascular changes in Nox5 mice were associated with increased vascular smooth muscle cell [Ca2+]i, increased reactive oxygen species and nitrotyrosine levels, and hyperphosphorylation of pro-contractile signaling molecules MLC20 (myosin light chain 20) and MYPT1 (myosin phosphatase target subunit 1). Blood pressure was similar in wild-type and Nox5 mice. Nox5 did not amplify angiotensin II effects. In R. prolixus, gastrointestinal smooth muscle contraction was blunted by Nox5 silencing, but not by VAS2870 (Nox1/2/4 inhibitor).
Conclusions: Nox5 is a pro-contractile Nox isoform important in redox-sensitive contraction. This involves calcium-calmodulin and endoplasmic reticulum-regulated mechanisms. Our findings define a novel function for vascular Nox5, linking calcium and reactive oxygen species to the pro-contractile molecular machinery in vascular smooth muscle cells.
Keywords: cell signaling; contraction; vascular biology.
© 2018 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley.
Figures






Similar articles
-
Central role of c-Src in NOX5- mediated redox signalling in vascular smooth muscle cells in human hypertension.Cardiovasc Res. 2022 Mar 25;118(5):1359-1373. doi: 10.1093/cvr/cvab171. Cardiovasc Res. 2022. PMID: 34320175 Free PMC article.
-
Reactive Oxygen-Forming Nox5 Links Vascular Smooth Muscle Cell Phenotypic Switching and Extracellular Vesicle-Mediated Vascular Calcification.Circ Res. 2020 Sep 11;127(7):911-927. doi: 10.1161/CIRCRESAHA.119.316159. Epub 2020 Jun 22. Circ Res. 2020. PMID: 32564697
-
Ca2+-Dependent NOX5 (NADPH Oxidase 5) Exaggerates Cardiac Hypertrophy Through Reactive Oxygen Species Production.Hypertension. 2020 Sep;76(3):827-838. doi: 10.1161/HYPERTENSIONAHA.120.15558. Epub 2020 Jul 20. Hypertension. 2020. PMID: 32683902
-
Vascular Biology of Superoxide-Generating NADPH Oxidase 5-Implications in Hypertension and Cardiovascular Disease.Antioxid Redox Signal. 2019 Mar 1;30(7):1027-1040. doi: 10.1089/ars.2018.7583. Epub 2018 Nov 15. Antioxid Redox Signal. 2019. PMID: 30334629 Free PMC article. Review.
-
NOX5: Molecular biology and pathophysiology.Exp Physiol. 2019 May;104(5):605-616. doi: 10.1113/EP086204. Epub 2019 Mar 18. Exp Physiol. 2019. PMID: 30801870 Free PMC article. Review.
Cited by
-
New Progress in the Molecular Regulations and Therapeutic Applications in Cardiac Oxidative Damage Caused by Pressure Overload.Antioxidants (Basel). 2022 Apr 29;11(5):877. doi: 10.3390/antiox11050877. Antioxidants (Basel). 2022. PMID: 35624741 Free PMC article. Review.
-
Non-immune Traits Triggered by Blood Intake Impact Vectorial Competence.Front Physiol. 2021 Mar 2;12:638033. doi: 10.3389/fphys.2021.638033. eCollection 2021. Front Physiol. 2021. PMID: 33737885 Free PMC article. Review.
-
Delineating the NOX-Mediated Promising Therapeutic Strategies for the Management of Various Cardiovascular Disorders: A Comprehensive Review.Curr Vasc Pharmacol. 2025;23(1):12-30. doi: 10.2174/0115701611308870240910115023. Curr Vasc Pharmacol. 2025. PMID: 39313896 Review.
-
Interrelation between ROS and Ca2+ in aging and age-related diseases.Redox Biol. 2020 Sep;36:101678. doi: 10.1016/j.redox.2020.101678. Epub 2020 Aug 7. Redox Biol. 2020. PMID: 32810740 Free PMC article. Review.
-
Nitric oxide and heme-NO stimulate superoxide production by NADPH oxidase 5.Free Radic Biol Med. 2021 Aug 20;172:252-263. doi: 10.1016/j.freeradbiomed.2021.06.008. Epub 2021 Jun 15. Free Radic Biol Med. 2021. PMID: 34139309 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

