Reorganization of Destabilized Nodes of Ranvier in β IV Spectrin Mutants Uncovers Critical Timelines for Nodal Restoration and Prevention of Motor Paresis
- PMID: 29907663
- PMCID: PMC6041796
- DOI: 10.1523/JNEUROSCI.0515-18.2018
Reorganization of Destabilized Nodes of Ranvier in β IV Spectrin Mutants Uncovers Critical Timelines for Nodal Restoration and Prevention of Motor Paresis
Abstract
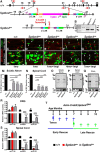
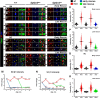
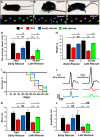
Disorganization of nodes of Ranvier is associated with motor and sensory dysfunctions. Mechanisms that allow nodal recovery during pathological processes remain poorly understood. A highly enriched nodal cytoskeletal protein βIV spectrin anchors and stabilizes the nodal complex to actin cytoskeleton. Loss of murine βIV spectrin allows the initial nodal organization, but causes gradual nodal destabilization. Mutations in human βIV spectrin cause auditory neuropathy and impairment in motor coordination. Similar phenotypes are caused by nodal disruption due to demyelination. Here we report on the precise timelines of nodal disorganization and reorganization by following disassembly and reassembly of key nodal proteins in βIV spectrin mice of both sexes before and after βIV spectrin re-expression at specifically chosen developmental time points. We show that the timeline of nodal restoration has different outcomes in the PNS and CNS with respect to nodal reassembly and functional restoration. In the PNS, restoration of nodes occurs within 1 month regardless of the time of βIV spectrin re-expression. In contrast, the CNS nodal reorganization and functional restoration occurs within a critical time window; after that, nodal reorganization diminishes, leading to less efficient motor recovery. We demonstrate that timely restoration of nodes can improve both the functional properties and the ultrastructure of myelinated fibers affected by long-term nodal disorganization. Our studies, which indicate a critical timeline for nodal restoration together with overall motor performance and prolonged life span, further support the idea that nodal restoration is more beneficial if initiated before any axonal damage, which is critically relevant to demyelinating disorders.SIGNIFICANCE STATEMENT Nodes of Ranvier are integral to efficient and rapid signal transmission along myelinated fibers. Various demyelinating disorders are characterized by destabilization of the nodal molecular complex, accompanied by severe reduction in nerve conduction and the onset of motor and sensory dysfunctions. This study is the first to report in vivo reassembly of destabilized nodes with sequential improvement in overall motor performance. Our study reveals that nodal restoration is achievable before any axonal damage, and that long-term nodal destabilization causes irreversible axonal structural changes that prevent functional restoration. Our studies provide significant insights into timely restoration of nodal domains as a potential therapeutic approach in treatment of demyelinating disorders.
Keywords: axonal health; motor coordination; myelination; nerve conduction; nodal restoration; nodes of Ranvier.
Copyright © 2018 the authors 0270-6474/18/386267-16$15.00/0.
Figures




Comment in
-
Nodal Dynamics after In Vivo Rescue of βIV Spectrin Expression.J Neurosci. 2019 Jan 2;39(1):15-17. doi: 10.1523/JNEUROSCI.2149-18.2018. J Neurosci. 2019. PMID: 30602540 Free PMC article. No abstract available.
References
-
- Bhat MA, Rios JC, Lu Y, Garcia-Fresco GP, Ching W, St Martin M, Li J, Einheber S, Chesler M, Rosenbluth J, Salzer JL, Bellen HJ (2001) Axon-glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron 30:369–383. 10.1016/S0896-6273(01)00294-X - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
