Exosomes Shuttle TREX1-Sensitive IFN-Stimulatory dsDNA from Irradiated Cancer Cells to DCs
- PMID: 29907693
- PMCID: PMC6072562
- DOI: 10.1158/2326-6066.CIR-17-0581
Exosomes Shuttle TREX1-Sensitive IFN-Stimulatory dsDNA from Irradiated Cancer Cells to DCs
Abstract
Radiotherapy (RT) used at immunogenic doses leads to accumulation of cytosolic double-stranded DNA (dsDNA) in cancer cells, which activates type I IFN (IFN-I) via the cGAS/STING pathway. Cancer cell-derived IFN-I is required to recruit BATF3-dependent dendritic cells (DC) to poorly immunogenic tumors and trigger antitumor T-cell responses in combination with immune checkpoint blockade. We have previously demonstrated that the exonuclease TREX1 regulates radiation immunogenicity by degrading cytosolic dsDNA. Tumor-derived DNA can also activate cGAS/STING-mediated production of IFN-I by DCs infiltrating immunogenic tumors. However, how DNA from cancer cells is transferred to the cytoplasm of DCs remains unclear. Here, we showed that tumor-derived exosomes (TEX) produced by irradiated mouse breast cancer cells (RT-TEX) transfer dsDNA to DCs and stimulate DC upregulation of costimulatory molecules and STING-dependent activation of IFN-I. In vivo, RT-TEX elicited tumor-specific CD8+ T-cell responses and protected mice from tumor development significantly better than TEX from untreated cancer cells in a prophylactic vaccination experiment. We demonstrated that the IFN-stimulatory dsDNA cargo of RT-TEX is regulated by TREX1 expression in the parent cells. Overall, these results identify RT-TEX as a mechanism whereby IFN-stimulatory dsDNA is transferred from irradiated cancer cells to DCs. We have previously shown that the expression of TREX1 is dependent on the RT dose size. Thus, these data have important implications for the use of RT with immunotherapy. Cancer Immunol Res; 6(8); 910-20. ©2018 AACR.
©2018 American Association for Cancer Research.
Conflict of interest statement
Figures
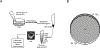
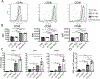
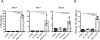


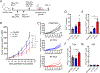
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

