DIACYLGLYCEROL ACYLTRANSFERASE1 Contributes to Freezing Tolerance
- PMID: 29907701
- PMCID: PMC6084661
- DOI: 10.1104/pp.18.00503
DIACYLGLYCEROL ACYLTRANSFERASE1 Contributes to Freezing Tolerance
Abstract
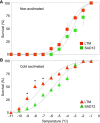
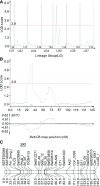
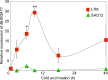
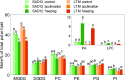
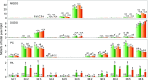
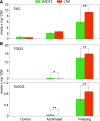
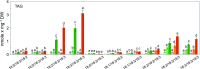
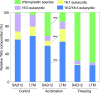
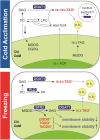
Freezing limits plant growth and crop productivity, and plant species in temperate zones have the capacity to develop freezing tolerance through complex modulation of gene expression affecting various aspects of metabolism and physiology. While many components of freezing tolerance have been identified in model species under controlled laboratory conditions, little is known about the mechanisms that impart freezing tolerance in natural populations of wild species. Here, we performed a quantitative trait locus (QTL) study of acclimated freezing tolerance in seedlings of Boechera stricta, a highly adapted relative of Arabidopsis (Arabidopsis thaliana) native to the Rocky Mountains. A single QTL was identified that contained the gene encoding ACYL-COENZYME A:DIACYLGLYCEROL ACYLTRANSFERASE1 (BstDGAT1), whose expression is highly cold responsive. The primary metabolic enzyme DGAT1 catalyzes the final step in assembly of triacylglycerol (TAG) by acyl transfer from acyl-CoA to diacylglycerol. Freezing tolerant plants showed higher DGAT1 expression during cold acclimation than more sensitive plants, and this resulted in increased accumulation of TAG in response to subsequent freezing. Levels of oligogalactolipids that are produced by SFR2 (SENSITIVE TO FREEZING2), an indispensable element of freezing tolerance in Arabidopsis, were also higher in freezing-tolerant plants. Furthermore, overexpression of AtDGAT1 led to increased freezing tolerance. We propose that DGAT1 confers freezing tolerance in plants by supporting SFR2-mediated remodeling of chloroplast membranes.
© 2018 American Society of Plant Biologists. All rights reserved.
Figures

Comment in
-
Baby, It's Cold Inside: Maintaining Membrane Integrity during Freezing.Plant Physiol. 2018 Aug;177(4):1350-1351. doi: 10.1104/pp.18.00809. Plant Physiol. 2018. PMID: 30087202 Free PMC article. No abstract available.
References
-
- Agarwal M, Hao Y, Kapoor A, Dong CH, Fujii H, Zheng X, Zhu JK (2006) A R2R3 type MYB transcription factor is involved in the cold regulation of CBF genes and in acquired freezing tolerance. J Biol Chem 281: 37636–37645 - PubMed
-
- Alonso-Blanco C, Gomez-Mena C, Llorente F, Koornneef M, Salinas J, Martínez-Zapater JM (2005) Genetic and molecular analyses of natural variation indicate CBF2 as a candidate gene for underlying a freezing tolerance quantitative trait locus in Arabidopsis. Plant Physiol 139: 1304–1312 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

