Dual-functional peptide with defective interfering genes effectively protects mice against avian and seasonal influenza
- PMID: 29907765
- PMCID: PMC6004018
- DOI: 10.1038/s41467-018-04792-7
Dual-functional peptide with defective interfering genes effectively protects mice against avian and seasonal influenza
Abstract
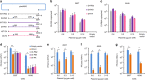
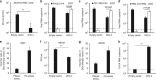
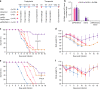
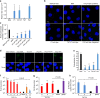
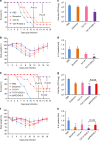
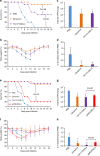
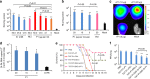
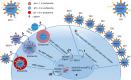
Limited efficacy of current antivirals and antiviral-resistant mutations impairs anti-influenza treatment. Here, we evaluate the in vitro and in vivo antiviral effect of three defective interfering genes (DIG-3) of influenza virus. Viral replication is significantly reduced in cell lines transfected with DIG-3. Mice treated with DIG-3 encoded by jetPEI-vector, as prophylaxis and therapeutics against A(H7N7) virus, respectively, have significantly better survivals (80% and 50%) than control mice (0%). We further develop a dual-functional peptide TAT-P1, which delivers DIG-3 with high efficiency and concomitantly exerts antiviral activity by preventing endosomal acidification. TAT-P1/DIG-3 is more effective than jetPEI/DIG-3 in treating A(H7N7) or A(H1N1)pdm09-infected mice and shows potent prophylactic protection on A(H7N7) or A(H1N1)pdm09-infected mice. The addition of P1 peptide, which prevents endosomal acidification, can enhance the protection of TAT-P1/DIG-3 on A(H1N1)pdm09-infected mice. Dual-functional TAT-P1 with DIG-3 can effectively protect or treat mice infected by avian and seasonal influenza virus.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
The NF-kappaB inhibitor SC75741 protects mice against highly pathogenic avian influenza A virus.Antiviral Res. 2013 Sep;99(3):336-44. doi: 10.1016/j.antiviral.2013.06.008. Epub 2013 Jun 28. Antiviral Res. 2013. PMID: 23811282
-
In vitro and in vivo efficacy of fluorodeoxycytidine analogs against highly pathogenic avian influenza H5N1, seasonal, and pandemic H1N1 virus infections.Antiviral Res. 2011 Nov;92(2):329-40. doi: 10.1016/j.antiviral.2011.09.001. Epub 2011 Sep 8. Antiviral Res. 2011. PMID: 21925541 Free PMC article.
-
Antiviral activity of SA-2 against influenza A virus in vitro/vivo and its inhibition of RNA polymerase.Antiviral Res. 2016 Mar;127:68-78. doi: 10.1016/j.antiviral.2016.01.011. Epub 2016 Jan 21. Antiviral Res. 2016. PMID: 26802558
-
3-Indoleacetonitrile Is Highly Effective in Treating Influenza A Virus Infection In Vitro and In Vivo.Viruses. 2021 Jul 23;13(8):1433. doi: 10.3390/v13081433. Viruses. 2021. PMID: 34452298 Free PMC article.
-
Adjunctive therapies and immunomodulating agents for severe influenza.Influenza Other Respir Viruses. 2013 Nov;7 Suppl 3(Suppl 3):52-9. doi: 10.1111/irv.12171. Influenza Other Respir Viruses. 2013. PMID: 24215382 Free PMC article. Review.
Cited by
-
Comparative Transcriptomic Analysis of Rhinovirus and Influenza Virus Infection.Front Microbiol. 2020 Jul 21;11:1580. doi: 10.3389/fmicb.2020.01580. eCollection 2020. Front Microbiol. 2020. PMID: 32849329 Free PMC article.
-
Single-Cell Analysis Uncovers a Vast Diversity in Intracellular Viral Defective Interfering RNA Content Affecting the Large Cell-to-Cell Heterogeneity in Influenza A Virus Replication.Viruses. 2020 Jan 7;12(1):71. doi: 10.3390/v12010071. Viruses. 2020. PMID: 31936115 Free PMC article.
-
Antiviral Activity of Influenza A Virus Defective Interfering Particles against SARS-CoV-2 Replication In Vitro through Stimulation of Innate Immunity.Cells. 2021 Jul 11;10(7):1756. doi: 10.3390/cells10071756. Cells. 2021. PMID: 34359926 Free PMC article.
-
Semi-continuous Propagation of Influenza A Virus and Its Defective Interfering Particles: Analyzing the Dynamic Competition To Select Candidates for Antiviral Therapy.J Virol. 2021 Nov 23;95(24):e0117421. doi: 10.1128/JVI.01174-21. Epub 2021 Sep 22. J Virol. 2021. PMID: 34550771 Free PMC article.
-
Defective Interfering Viral Particle Treatment Reduces Clinical Signs and Protects Hamsters from Lethal Nipah Virus Disease.mBio. 2022 Apr 26;13(2):e0329421. doi: 10.1128/mbio.03294-21. Epub 2022 Mar 17. mBio. 2022. PMID: 35297677 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

