Ex vivo biomechanical characterization of syringe-needle ejections for intracerebral cell delivery
- PMID: 29907825
- PMCID: PMC6004017
- DOI: 10.1038/s41598-018-27568-x
Ex vivo biomechanical characterization of syringe-needle ejections for intracerebral cell delivery
Abstract
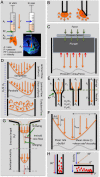
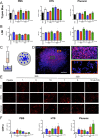
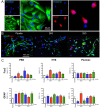
Intracerebral implantation of cell suspensions is finding its clinical translation with encouraging results in patients with stroke. However, the survival of cells in the brain remains poor. Although the biological potential of neural stem cells (NSCs) is widely documented, the biomechanical effects of delivering cells through a syringe-needle remain poorly understood. We here detailed the biomechanical forces (pressure, shear stress) that cells are exposed to during ejection through different sized needles (20G, 26G, 32G) and syringes (10, 50, 250 µL) at relevant flow rates (1, 5, 10 µL/min). A comparison of 3 vehicles, Phosphate Buffered Saline (PBS), Hypothermosol (HTS), and Pluronic, indicated that less viscous vehicles are favorable for suspension with a high cell volume fraction to minimize sedimentation. Higher suspension viscosity was associated with greater shear stress. Higher flow rates with viscous vehicle, such as HTS reduced viability by ~10% and also produced more apoptotic cells (28%). At 5 µL/min ejection using a 26G needle increased neuronal differentiation for PBS and HTS suspensions. These results reveal the biological impact of biomechanical forces in the cell delivery process. Appropriate engineering strategies can be considered to mitigate these effects to ensure the efficacious translation of this promising therapy.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Intracerebral Cell Implantation: Preparation and Characterization of Cell Suspensions.Cell Transplant. 2016;25(4):645-64. doi: 10.3727/096368915X690350. Epub 2015 Dec 30. Cell Transplant. 2016. PMID: 26720923
-
Hydrogel encapsulation to improve cell viability during syringe needle flow.J Long Term Eff Med Implants. 2014;24(2-3):151-62. doi: 10.1615/jlongtermeffmedimplants.2014010946. J Long Term Eff Med Implants. 2014. PMID: 25272214
-
The Association between Needle Size and Waste Product and Its Effect on Cost-Effectiveness of Botulinum Toxin Injections?Facial Plast Surg. 2020 Aug;36(4):484-486. doi: 10.1055/s-0040-1713793. Epub 2020 Jul 20. Facial Plast Surg. 2020. PMID: 32688397
-
Magnetic Resonance Imaging-Guided Delivery of Neural Stem Cells into the Basal Ganglia of Nonhuman Primates Reveals a Pulsatile Mode of Cell Dispersion.Stem Cells Transl Med. 2017 Mar;6(3):877-885. doi: 10.5966/sctm.2016-0269. Epub 2016 Sep 22. Stem Cells Transl Med. 2017. PMID: 28297573 Free PMC article.
-
How to use stem cells for repair in stroke patients.Rev Neurol (Paris). 2017 Nov;173(9):572-576. doi: 10.1016/j.neurol.2017.09.003. Epub 2017 Oct 21. Rev Neurol (Paris). 2017. PMID: 29033030 Review.
Cited by
-
Method of long-term, recurrent, intracerebroventricular infusion of cellular therapy in mice.J Neurosci Methods. 2022 Apr 1;371:109529. doi: 10.1016/j.jneumeth.2022.109529. Epub 2022 Feb 17. J Neurosci Methods. 2022. PMID: 35183615 Free PMC article. No abstract available.
-
Cytoprotection of Human Progenitor and Stem Cells through Encapsulation in Alginate Templated, Dual Crosslinked Silk and Silk-Gelatin Composite Hydrogel Microbeads.Adv Healthc Mater. 2022 Sep;11(17):e2200293. doi: 10.1002/adhm.202200293. Epub 2022 Jun 22. Adv Healthc Mater. 2022. PMID: 35686928 Free PMC article.
-
3D-Printed Microinjection Needle Arrays via a Hybrid DLP-Direct Laser Writing Strategy.Adv Mater Technol. 2023 Mar 10;8(5):2201641. doi: 10.1002/admt.202201641. Epub 2023 Feb 5. Adv Mater Technol. 2023. PMID: 37064271 Free PMC article.
-
Impact of Needle Selection on Survival of Muscle-Derived Cells When Used for Laryngeal Injections.J Cell Sci Ther. 2023;14(1):377. Epub 2022 Dec 9. J Cell Sci Ther. 2023. PMID: 37250272 Free PMC article.
-
Human induced pluripotent stem cell-derived therapies for regeneration after central nervous system injury.Neural Regen Res. 2025 Nov 1;20(11):3063-3075. doi: 10.4103/NRR.NRR-D-24-00901. Epub 2024 Dec 16. Neural Regen Res. 2025. PMID: 39715081 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

