Deletion of DDB1- and CUL4- associated factor-17 (Dcaf17) gene causes spermatogenesis defects and male infertility in mice
- PMID: 29907856
- PMCID: PMC6003934
- DOI: 10.1038/s41598-018-27379-0
Deletion of DDB1- and CUL4- associated factor-17 (Dcaf17) gene causes spermatogenesis defects and male infertility in mice
Erratum in
-
Publisher Correction: Deletion of DDB1- and CUL4- associated factor-17 (Dcaf17) gene causes spermatogenesis defects and male infertility in mice.Sci Rep. 2018 Aug 1;8(1):11779. doi: 10.1038/s41598-018-29836-2. Sci Rep. 2018. PMID: 30068992 Free PMC article.
Abstract
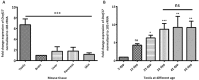
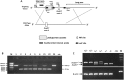
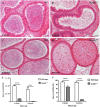
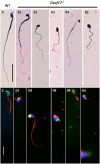
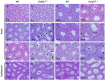
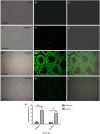
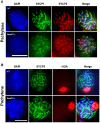
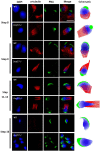
DDB1- and CUL4-associated factor 17 (Dcaf17) is a member of DCAF family genes that encode substrate receptor proteins for Cullin-RING E3 ubiquitin ligases, which play critical roles in many cellular processes. To unravel the function of DCAF17, we performed expression profiling of Dcaf17 in different tissues of wild type mouse by qRT-PCR and generated Dcaf17 knockout mice by gene targeting. Expression profiling of Dcaf17 showed highest expression in testis. Analyses of Dcaf17 transcripts during post-natal development of testis at different ages displayed gradual increase in Dcaf17 mRNA levels with the age. Although Dcaf17 disruption did not have any effect on female fertility, Dcaf17 deletion led to male infertility due to abnormal sperm development. The Dcaf17 -/- mice produced low number of sperm with abnormal shape and significantly low motility. Histological examination of the Dcaf17 -/- testis revealed impaired spermatogenesis with presence of vacuoles and sloughed cells in the seminiferous tubules. Disruption of Dcaf17 caused asymmetric acrosome capping, impaired nuclear compaction and abnormal round spermatid to elongated spermatid transition. For the first time, these data indicate that DCAF17 is essential for spermiogenesis.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

