Attenuation of Retinal Vascular Development in Neonatal Mice Subjected to Hypoxic-Ischemic Encephalopathy
- PMID: 29907863
- PMCID: PMC6003906
- DOI: 10.1038/s41598-018-27525-8
Attenuation of Retinal Vascular Development in Neonatal Mice Subjected to Hypoxic-Ischemic Encephalopathy
Abstract
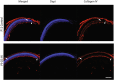
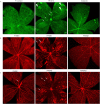
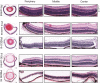
A significant proportion of children that survive hypoxic-ischemic encephalopathy (HIE) develop visual impairment. These visual deficits are generally attributed to injuries that occur in the primary visual cortex and other visual processing systems. Recent studies suggested that neuronal damage might also occur in the retina. An important structure affecting the viability of retinal neurons is the vasculature. However, the effects of HIE on the retinal neurovasculature have not been systemically evaluated. Here we investigated whether exposure of postnatal day 9 (P9) neonatal mice to HIE is sufficient to induce neurovascular damage in the retina. We demonstrate that the blood vessels on the surface of the retina, from mice subjected to HIE, were abnormally enlarged with signs of degeneration. The intermediate and deep vascular layers in these retinas failed to form normally, particularly in the periphery. All the vascular damages observed here were irreversible in nature up to 100 days post HIE. We also observed loss of retinal neurons, together with changes in both astrocytes and Müller cells mainly in the inner retina at the periphery. Collectively, our findings suggest that HIE results in profound alterations in the retinal vasculature, indicating the importance of developing therapeutic strategies to protect neurovascular dysfunction not only in the brain but also in the retina for infants exposed to HIE.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Mezer E, et al. Trends in the incidence and causes of severe visual impairment and blindness in children from Israel. Journal of AAPOS: the official publication of the American Association for Pediatric Ophthalmology and Strabismus. 2015;19:260–265.e261. doi: 10.1016/j.jaapos.2015.04.002. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

