The current state of the art for biological therapies and new small molecules in inflammatory bowel disease
- PMID: 29907872
- PMCID: PMC6279599
- DOI: 10.1038/s41385-018-0050-3
The current state of the art for biological therapies and new small molecules in inflammatory bowel disease
Abstract
The emergence of biologic therapies is arguably the greatest therapeutic advance in the care of inflammatory bowel disease (IBD) to date, allowing directed treatments targeted at highly specific molecules shown to play critical roles in disease pathogenesis, with advantages in potency and selectivity. Furthermore, a large number of new biologic and small-molecule therapies in IBD targeting a variety of pathways are at various stages of development that should soon lead to a dramatic expansion in our therapeutic armamentarium. Additionally, since the initial introduction of biologics, there have been substantial advances in our understanding as to how biologics work, the practical realities of their administration, and how to enhance their efficacy and safety in the clinical setting. In this review, we will summarize the current state of the art for biological therapies in IBD, both in terms of agents available and their optimal use, as well as preview future advances in biologics and highly targeted small molecules in the IBD field.
Conflict of interest statement
Figures

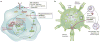
References
-
- Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. - PubMed
-
- Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn’s disease. Lancet. 2017;389:1741–1755. - PubMed
-
- Gomollon F, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis. 2017;11:3–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

