Semaglutide s.c. Once-Weekly in Type 2 Diabetes: A Population Pharmacokinetic Analysis
- PMID: 29907893
- PMCID: PMC6064581
- DOI: 10.1007/s13300-018-0458-5
Semaglutide s.c. Once-Weekly in Type 2 Diabetes: A Population Pharmacokinetic Analysis
Abstract
Introduction: Semaglutide, a new treatment option approved for the treatment of patients with type 2 diabetes mellitus, is a glucagon-like peptide-1 receptor agonist to be injected subcutaneously once weekly. This analysis used a population pharmacokinetic model of semaglutide to identify clinically relevant covariates for exposure.
Methods: A total of 1612 patients with up to seven pharmacokinetic observations each were included in the analysis. All subjects had type 2 diabetes mellitus and were enrolled in one of five trials in the phase III development program for subcutaneous semaglutide once weekly (the SUSTAIN program). The treatment duration of the trials varied from 30 to 104 weeks.
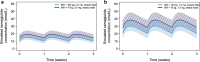
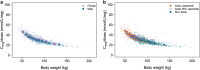
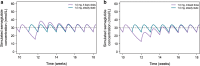
Results: No clinically relevant effects on the exposure were seen for sex, age, race, ethnicity, renal function, or injection site used, and semaglutide exposure was stable over time. Of the covariates chosen, only body weight had a relevant effect on the exposure of semaglutide. Few subjects developed semaglutide antibodies, and the antibodies had no effect on exposure. Dose proportionality was shown for the 0.5 mg and 1.0 mg maintenance doses of semaglutide.
Conclusion: The population pharmacokinetic study showed that semaglutide exposure is not affected by covariates other than body weight at either a maintenance dose of 0.5 or 1.0 mg semaglutide. Therefore, we conclude that no semaglutide dose adjustments are needed in different populations. This finding is to be further explored in an exposure-response analysis.
Trial registration: The trials were registered at ClinicalTrials.gov (identifiers: NCT02054897, NCT01930188, NCT01885208, NCT01720446 and NCT02207374).
Funding: Novo Nordisk A/S, Bagsværd, Denmark.
Keywords: GLP-1 analog; GLP-1 receptor agonist; Population pharmacokinetics; Semaglutide.
Figures


References
-
- International Diabetes Federation . IDF diabetes atlas. 7. Brussels: International Diabetes Federation; 2015. - PubMed
-
- Noto H, Tsujimoto T, Sasazuki T, Noda M. Significantly increased risk of cancer in patients with diabetes mellitus: a systematic review and meta-analysis. EndocrPract. 2011;17:616–628. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

