Exosomes in cancer development and clinical applications
- PMID: 29908100
- PMCID: PMC6113508
- DOI: 10.1111/cas.13697
Exosomes in cancer development and clinical applications
Abstract
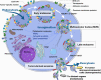
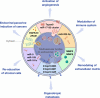
Exosomes participate in cancer progression and metastasis by transferring bioactive molecules between cancer and various cells in the local and distant microenvironments. Such intercellular cross-talk results in changes in multiple cellular and biological functions in recipient cells. Several hallmarks of cancer have reportedly been impacted by this exosome-mediated cell-to-cell communication, including modulating immune responses, reprogramming stromal cells, remodeling the architecture of the extracellular matrix, or even endowing cancer cells with characteristics of drug resistance. Selectively, loading specific oncogenic molecules into exosomes highlights exosomes as potential diagnostic biomarkers as well as therapeutic targets. In addition, exosome-based drug delivery strategies in preclinical and clinical trials have been shown to dramatically decrease cancer development. In the present review, we summarize the significant aspects of exosomes in cancer development that can provide novel strategies for potential clinical applications.
Keywords: biomarker; cancer malignancy; cancer therapy; drug resistance; exosome.
© 2018 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

