Leveraging the Knorr Pyrazole Synthesis for the Facile Generation of Thioester Surrogates for use in Native Chemical Ligation
- PMID: 29908104
- PMCID: PMC6126375
- DOI: 10.1002/anie.201805191
Leveraging the Knorr Pyrazole Synthesis for the Facile Generation of Thioester Surrogates for use in Native Chemical Ligation
Abstract
Facile synthesis of C-terminal thioesters is integral to native chemical ligation (NCL) strategies for chemical protein synthesis. We introduce a new method of mild peptide activation, which leverages solid-phase peptide synthesis (SPPS) on an established resin linker and classical heterocyclic chemistry to convert C-terminal peptide hydrazides into their corresponding thioesters via an acyl pyrazole intermediate. Peptide hydrazides, synthesized on established trityl chloride resins, can be activated in solution with stoichiometric acetyl acetone (acac), readily proceed to the peptide acyl pyrazoles. Acyl pyrazoles are mild acylating agents and are efficiently exchanged with an aryl thiol, which can then be directly utilized in NCL. The mild, chemoselective, and stoichiometric activating conditions allow this method to be utilized through multiple sequential ligations without intermediate purification steps.
Keywords: chemoselective ligation; native chemical ligation; peptide hydrazides; protein synthesis; pyrazoles.
© 2018 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures


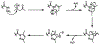



References
-
- Dawson PE, Muir TW, Clark-Lewis I, Kent SB, Science 1994, 266, 776–779. - PubMed
-
- Schnölzer M, Alewood P, Jones A, Alewood D, Kent SBH, Int. J. Pept. Res. Ther 2007, 13, 31–44.
-
- Muttenthaler M, Albericio F, Dawson PE, Nat. Protoc 2015, 10, 1067–1083. - PubMed
-
- Camarero Julio A. and Mitchell Alexander R., Protein Pept. Lett 2005, 12, 723–728. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

