PPAR-gamma pathways attenuate pulmonary granuloma formation in a carbon nanotube induced murine model of sarcoidosis
- PMID: 29908181
- PMCID: PMC6432932
- DOI: 10.1016/j.bbrc.2018.06.061
PPAR-gamma pathways attenuate pulmonary granuloma formation in a carbon nanotube induced murine model of sarcoidosis
Abstract
Peroxisome proliferator activated receptor gamma (PPARγ), a ligand activated nuclear transcription factor, is constitutively expressed in alveolar macrophages of healthy individuals. PPARγ deficiencies have been noted in several lung diseases including the alveolar macrophages of pulmonary sarcoidosis patients. We have previously described a murine model of multiwall carbon nanotubes (MWCNT) induced pulmonary granulomatous inflammation which bears striking similarities to pulmonary sarcoidosis, including the deficiency of alveolar macrophage PPARγ. Further studies demonstrate alveolar macrophage PPARγ deficiency exacerbates MWCNT-induced pulmonary granulomas. Based on these observations we hypothesized that activation of PPARγ via administration of the PPARγ-specific ligand rosiglitazone would limit MWCNT-induced granuloma formation and promote PPARγ-dependent pathways. Results presented here show that rosiglitazone significantly limits the frequency and severity of MWCNT-induced pulmonary granulomas. Furthermore, rosiglitazone attenuates alveolar macrophage NF-κB activity and downregulates the expression of the pro-inflammatory mediators, CCL2 and osteopontin. PPARγ activation via rosiglitazone also prevents the MWCNT-induced deficiency of PPARγ-regulated ATP-binding cassette lipid transporter-G1 (ABCG1) expression. ABCG1 is crucial to pulmonary lipid homeostasis. ABCG1 deficiency results in lipid accumulation which promotes pro-inflammatory macrophage activation. Our results indicate that restoration of homeostatic ABCG1 levels by rosiglitazone correlates with both reduced pulmonary lipid accumulation, and decreased alveolar macrophage activation. These data confirm and further support our previous observations that PPARγ pathways are critical in regulating MWCNT-induced pulmonary granulomatous inflammation.
Keywords: Alveolar macrophage; Carbon nanotube; Granuloma; Inflammation; Lipid transporters; Sarcoidosis.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures
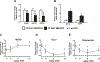
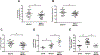
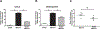
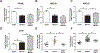
Similar articles
-
Studies in a Murine Granuloma Model of Instilled Carbon Nanotubes: Relevance to Sarcoidosis.Int J Mol Sci. 2021 Apr 2;22(7):3705. doi: 10.3390/ijms22073705. Int J Mol Sci. 2021. PMID: 33918196 Free PMC article. Review.
-
Peroxisome Proliferator-activated Receptor-γ Deficiency Exacerbates Fibrotic Response to Mycobacteria Peptide in Murine Sarcoidosis Model.Am J Respir Cell Mol Biol. 2019 Aug;61(2):198-208. doi: 10.1165/rcmb.2018-0346OC. Am J Respir Cell Mol Biol. 2019. PMID: 30741559 Free PMC article.
-
The role of PPARγ in carbon nanotube-elicited granulomatous lung inflammation.Respir Res. 2013 Jan 23;14(1):7. doi: 10.1186/1465-9921-14-7. Respir Res. 2013. PMID: 23343389 Free PMC article.
-
Elevated MicroRNA-33 in Sarcoidosis and a Carbon Nanotube Model of Chronic Granulomatous Disease.Am J Respir Cell Mol Biol. 2016 Jun;54(6):865-71. doi: 10.1165/rcmb.2015-0332OC. Am J Respir Cell Mol Biol. 2016. PMID: 26641802 Free PMC article.
-
Peroxisome proliferator-activated receptor gamma as modulator of inflammation in pulmonary sarcoidosis.Srp Arh Celok Lek. 2013 Sep-Oct;141(9-10):705-9. doi: 10.2298/sarh1310705p. Srp Arh Celok Lek. 2013. PMID: 24364239 Review.
Cited by
-
Advance in pathogenesis of sarcoidosis: Triggers and progression.Heliyon. 2024 Mar 8;10(5):e27612. doi: 10.1016/j.heliyon.2024.e27612. eCollection 2024 Mar 15. Heliyon. 2024. PMID: 38486783 Free PMC article. Review.
-
Pulmonary Sarcoidosis: Experimental Models and Perspectives of Molecular Diagnostics Using Quantum Dots.Int J Mol Sci. 2023 Jul 10;24(14):11267. doi: 10.3390/ijms241411267. Int J Mol Sci. 2023. PMID: 37511027 Free PMC article. Review.
-
The M2a Macrophage Phenotype Accompanies Pulmonary Granuloma Resolution in Mmp12 Knock-Out Mice Instilled with Multiwall Carbon Nanotubes.Int J Mol Sci. 2021 Oct 13;22(20):11019. doi: 10.3390/ijms222011019. Int J Mol Sci. 2021. PMID: 34681679 Free PMC article.
-
Alveolar macrophages: guardians of the alveolar lipid galaxy.Curr Opin Lipidol. 2025 Jun 1;36(3):153-162. doi: 10.1097/MOL.0000000000000987. Epub 2025 Apr 2. Curr Opin Lipidol. 2025. PMID: 40183504 Free PMC article. Review.
-
Studies in a Murine Granuloma Model of Instilled Carbon Nanotubes: Relevance to Sarcoidosis.Int J Mol Sci. 2021 Apr 2;22(7):3705. doi: 10.3390/ijms22073705. Int J Mol Sci. 2021. PMID: 33918196 Free PMC article. Review.
References
-
- Baughman RP, Teirstein AS, Judson MA, Rossman MD, Yeager H, Bresnitz EA, DePalo L, Hunninghake G, Iannuzzi MC, Johns CJ, McLennan G, Moller DR, Newman LS, Rabin DL, Rose C, Rybicki B, Weinberger SE, Terrin ML, Knatterud GL, Cherniak R, Clinical characteristics of patients in a case control study of sarcoidosis, Am. J. Respir. Crit. Care Med. 164 (2001) 1885–1889. - PubMed
-
- Chen ES, Moller DR, Etiology of sarcoidosis, Clin. Chest Med. 29 (2008) 365–377 vii. - PubMed
-
- Kajdasz DK, Lackland DT, Mohr LC, Judson MA, A current assessment of rurally linked exposures as potential risk factors for sarcoidosis, Ann. Epidemiol. 11 (2001) 111–117. - PubMed
-
- Kreider ME, Christie JD, Thompson B, Newman L, Rose C, Barnard J, Bresnitz E, Judson MA, Lackland DT, Rossman MD, Relationship of environmental exposures to the clinical phenotype of sarcoidosis, Chest 128 (2005) 207–215. - PubMed
-
- Prezant DJ, Dhala A, Goldstein A, Janus D, Ortiz F, Aldrich TK, Kelly KJ, The incidence, prevalence, and severity of sarcoidosis in New York City firefighters, Chest 116 (1999) 1183–1193. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

