Hydrogen sulfide epigenetically mitigates bone loss through OPG/RANKL regulation during hyperhomocysteinemia in mice
- PMID: 29908298
- PMCID: PMC6084464
- DOI: 10.1016/j.bone.2018.06.009
Hydrogen sulfide epigenetically mitigates bone loss through OPG/RANKL regulation during hyperhomocysteinemia in mice
Abstract
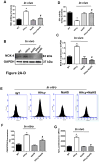
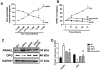
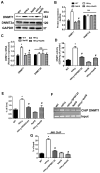
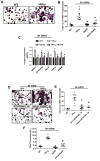
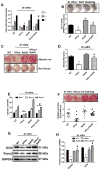
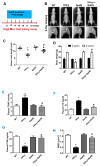
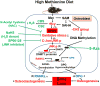
Hydrogen sulfide (H2S) is a novel gasotransmitter produced endogenously in mammalian cells, which works by mediating diverse physiological functions. An imbalance in H2S metabolism is associated with defective bone homeostasis. However, it is unknown whether H2S plays any epigenetic role in bone loss induced by hyperhomocysteinemia (HHcy). We demonstrate that diet-induced HHcy, a mouse model of metabolite induced osteoporosis, alters homocysteine metabolism by decreasing plasma levels of H2S. Treatment with NaHS (H2S donor), normalizes the plasma level of H2S and further alleviates HHcy induced trabecular bone loss and mechanical strength. Mechanistic studies have shown that DNMT1 expression is higher in the HHcy condition. The data show that activated phospho-JNK binds to the DNMT1 promoter and causes epigenetic DNA hyper-methylation of the OPG gene. This leads to activation of RANKL expression and mediates osteoclastogenesis. However, administration of NaHS could prevent HHcy induced bone loss. Therefore, H2S could be used as a novel therapy for HHcy mediated bone loss.
Keywords: Bone loss; DNA methyltransferase; Epigenetic DNA methylation; Osteoblastogenesis; Osteoclastogenesis.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
All authors declare no conflict of interest.
Figures







References
-
- Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289:1508–14. - PubMed
-
- Liu HY, Wu AT, Tsai CY, et al. The balance between adipogenesis and osteogenesis in bone regeneration by platelet-rich plasma for age-related osteoporosis. Biomaterials. 2011;32:6773–80. - PubMed
-
- Verron E, Gauthier O, Janvier P, et al. In vivo bone augmentation in an osteoporotic environment using bisphosphonate-loaded calcium deficient apatite. Biomaterials. 2010;31:7776–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

