Pathway-based predictive approaches for non-animal assessment of acute inhalation toxicity
- PMID: 29908304
- PMCID: PMC6760245
- DOI: 10.1016/j.tiv.2018.06.009
Pathway-based predictive approaches for non-animal assessment of acute inhalation toxicity
Abstract
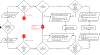
New approaches are needed to assess the effects of inhaled substances on human health. These approaches will be based on mechanisms of toxicity, an understanding of dosimetry, and the use of in silico modeling and in vitro test methods. In order to accelerate wider implementation of such approaches, development of adverse outcome pathways (AOPs) can help identify and address gaps in our understanding of relevant parameters for model input and mechanisms, and optimize non-animal approaches that can be used to investigate key events of toxicity. This paper describes the AOPs and the toolbox of in vitro and in silico models that can be used to assess the key events leading to toxicity following inhalation exposure. Because the optimal testing strategy will vary depending on the substance of interest, here we present a decision tree approach to identify an appropriate non-animal integrated testing strategy that incorporates consideration of a substance's physicochemical properties, relevant mechanisms of toxicity, and available in silico models and in vitro test methods. This decision tree can facilitate standardization of the testing approaches. Case study examples are presented to provide a basis for proof-of-concept testing to illustrate the utility of non-animal approaches to inform hazard identification and risk assessment of humans exposed to inhaled substances.
Keywords: Acute inhalation toxicity; Adverse outcome pathway; Aggregate exposure pathway; Dosimetry; Ex vivo; In silico; In vitro; Integrated approach to testing and assessment (IATA); Quantitative structure-activity relationships (QSAR); Risk assessment.
Copyright © 2018 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures
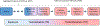

References
-
- 40 CFR 799.9130. (2002). “TSCA Acute Inhalation Toxicity.” from https://www.gpo.gov/fdsys/search/pagedetails.action?packageId=CFR-2002-t....
-
- Allen TE, Goodman JM, et al. (2014). “Defining molecular initiating events in the adverse outcome pathway framework for risk assessment.” Chemical Research in Toxicology 27(12): 2100–2112. - PubMed
-
- Ankley GT, Bennett RS, et al. (2010). “Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment.” Environmental Toxicology and Chemistry 29(3): 730–741. - PubMed
-
- Armitage JM, Wania F, et al. (2014). “Application of mass balance models and the chemical activity concept to facilitate the use of in vitro toxicity data for risk assessment.” Environ Sci Technol 48(16): 9770–9779. - PubMed
-
- Balls M (1991). “Why modification of the LD50 test will not be enough.” Laboratory Animals 25: 198–206. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

