Disruption of gul-1 decreased the culture viscosity and improved protein secretion in the filamentous fungus Neurospora crassa
- PMID: 29908565
- PMCID: PMC6004096
- DOI: 10.1186/s12934-018-0944-5
Disruption of gul-1 decreased the culture viscosity and improved protein secretion in the filamentous fungus Neurospora crassa
Abstract
Background: The cellulolytic fungus Neurospora crassa is considered a potential host for enzyme and bioethanol production. However, large scale applications are hindered by its filamentous growth. Although previous investigations have shown that mycelial morphology in submerged culture can be controlled by altering physical factors, there is little knowledge available about the potential for morphology control by genetic modification.
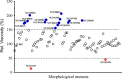
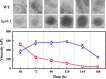
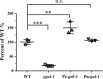
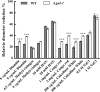
Results: In this study, we screened morphological mutants in the filamentous fungus N. crassa. Of the 90 morphological mutants screened, 14 mutants exhibited considerably higher viscosity compared with that of the wild type strain, and only two mutants showed low-viscosity morphologies in submerged culture. We observed that disruption of gul-1 (NCU01197), which encodes an mRNA binding protein involved in cell wall remodeling, caused pellet formation as the fermentation progressed, and resulted in the most significant decrease in viscosity of culture broth. Moreover, over-expression of gul-1 caused dramatically increased viscosity, suggesting that the gul-1 had an important function in mycelial morphology during submerged cultivation. Transcriptional profiling showed that expression of genes encoding eight GPI-anchored cell wall proteins was lowered in Δgul-1 while expression of genes associated with two non-anchored cell wall proteins was elevated. Meanwhile, the expression levels of two hydrophobin genes were also significantly altered. These results suggested that GUL-1 affected the transcription of cell wall-related genes, thereby influencing cell wall structure and mycelial morphology. Additionally, the deletion of gul-1 caused increased protein secretion, probably due to a defect in cell wall integrity, suggesting this as an alternative strategy of strain improvement for enzyme production. To confirm practical applications, deletion of gul-1 in the hyper-cellulase producing strain (∆ncw-1∆Ncap3m) significantly reduced the viscosity of culture broth.
Conclusions: Using the model filamentous fungus N. crassa, genes that affect mycelial morphology in submerged culture were explored through systematic screening of morphological mutants. Disrupting several candidate genes altered viscosities in submerged culture. This work provides an example for controlling fungal morphology in submerged fermentation by genetic engineering, and will be beneficial for industrial fungal strain improvement.
Keywords: Mycelial morphology; Neurospora crassa; Pellet; Protein secretion; Viscosity.
Figures



Similar articles
-
[Improving cellulases production with Neurospora crassa by morphology mutants screening].Sheng Wu Gong Cheng Xue Bao. 2014 Jan;30(1):55-63. Sheng Wu Gong Cheng Xue Bao. 2014. PMID: 24818479 Chinese.
-
Transcriptomic and genetic analysis reveals a Zn2Cys6 transcription factor specifically required for conidiation in submerged cultures of Thermothelomyces thermophilus.mBio. 2025 Jan 8;16(1):e0311124. doi: 10.1128/mbio.03111-24. Epub 2024 Nov 27. mBio. 2025. PMID: 39601596 Free PMC article.
-
Regulation of Neurospora crassa cell wall remodeling via the cot-1 pathway is mediated by gul-1.Curr Genet. 2017 Feb;63(1):145-159. doi: 10.1007/s00294-016-0625-z. Epub 2016 Jun 30. Curr Genet. 2017. PMID: 27363849
-
Biotechnological production of ethanol from renewable resources by Neurospora crassa: an alternative to conventional yeast fermentations?Appl Microbiol Biotechnol. 2013 Feb;97(4):1457-73. doi: 10.1007/s00253-012-4655-2. Epub 2013 Jan 15. Appl Microbiol Biotechnol. 2013. PMID: 23318834 Review.
-
The Genetics and Biochemistry of Cell Wall Structure and Synthesis in Neurospora crassa, a Model Filamentous Fungus.Front Microbiol. 2019 Oct 10;10:2294. doi: 10.3389/fmicb.2019.02294. eCollection 2019. Front Microbiol. 2019. PMID: 31649638 Free PMC article. Review.
Cited by
-
STK-12 acts as a transcriptional brake to control the expression of cellulase-encoding genes in Neurospora crassa.PLoS Genet. 2019 Nov 25;15(11):e1008510. doi: 10.1371/journal.pgen.1008510. eCollection 2019 Nov. PLoS Genet. 2019. PMID: 31765390 Free PMC article.
-
From induction to secretion: a complicated route for cellulase production in Trichoderma reesei.Bioresour Bioprocess. 2021 Oct 22;8(1):107. doi: 10.1186/s40643-021-00461-8. Bioresour Bioprocess. 2021. PMID: 38650205 Free PMC article. Review.
-
Disruption of the Trichoderma reesei gul1 gene stimulates hyphal branching and reduces broth viscosity in cellulase production.J Ind Microbiol Biotechnol. 2021 Apr 30;48(1-2):kuab012. doi: 10.1093/jimb/kuab012. J Ind Microbiol Biotechnol. 2021. PMID: 33693788 Free PMC article.
-
RNAi-Mediated Gene Silencing of Trcot1 Induces a Hyperbranching Phenotype in Trichoderma reesei.J Microbiol Biotechnol. 2020 Feb 28;30(2):206-215. doi: 10.4014/jmb.1909.09050. J Microbiol Biotechnol. 2020. PMID: 31752060 Free PMC article.
-
Quantitative Proteome Profiling Reveals Cellobiose-Dependent Protein Processing and Export Pathways for the Lignocellulolytic Response in Neurospora crassa.Appl Environ Microbiol. 2020 Jul 20;86(15):e00653-20. doi: 10.1128/AEM.00653-20. Print 2020 Jul 20. Appl Environ Microbiol. 2020. PMID: 32471912 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

