Adropin regulates pyruvate dehydrogenase in cardiac cells via a novel GPCR-MAPK-PDK4 signaling pathway
- PMID: 29909017
- PMCID: PMC6008287
- DOI: 10.1016/j.redox.2018.06.003
Adropin regulates pyruvate dehydrogenase in cardiac cells via a novel GPCR-MAPK-PDK4 signaling pathway
Abstract
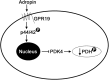
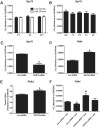
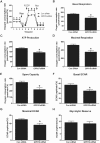
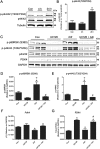
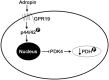
Mitochondria supply ~90% of the ATP required for contractile function in cardiac cells. While adult cardiomyocytes preferentially utilize fatty acids as a fuel source for oxidative phosphorylation, cardiac mitochondria can switch to other substrates when required. This change is driven in part by a combination of extracellular and intracellular signal transduction pathways that alter mitochondrial gene expression and enzymatic activity. The mechanisms by which extracellular metabolic information is conveyed to cardiac mitochondria are not currently well defined. Recent work has shown that adropin - a liver-secreted peptide hormone - can induce changes in mitochondrial fuel substrate utilization in skeletal muscle, leading to increased glucose use. In this study, we examined whether adropin could regulate mitochondrial glucose utilization pathways in cardiac cells. We show that stimulation of cultured cardiac cells with adropin leads to decreased expression of the pyruvate dehydrogenase (PDH) negative regulator PDK4, which reduces inhibitory PDH phosphorylation. The downregulation of PDK4 expression by adropin is lost when GPR19 - a putative adropin receptor - is genetically depleted in H9c2 cells. Loss of GRP19 expression alone increased PDK4 expression, leading to a reduction in mitochondrial respiration. Finally, we show that adropin-mediated GPR19 signaling relies on the p44/42 MAPK pathway, and that pharmacological disruption of this pathway blocks the effects of adropin on PDK4 in cardiac cells. These findings suggest that adropin may be a key regulator of fuel substrate utilization in the heart, and implicates an orphan G-protein coupled receptor in a novel signaling pathway controlling mitochondrial fuel metabolism.
Keywords: Adropin; GPCR; GRP19; Metabolism; Mitochondria; PDK4; Pyruvate dehydrogenase.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Figures


References
-
- Cornall L.M., Mathai M.L., Hryciw D.H., Simcocks A.C., O'Brien P.E., Wentworth J.M., McAinch A.J. GPR119 regulates genetic markers of fatty acid oxidation in cultured skeletal muscle myotubes. Mol. Cell. Endocrinol. 2013;365:108–118. - PubMed
-
- Fujie S., Hasegawa N., Sato K., Fujita S., Sanada K., Hamaoka T., Iemitsu M. Aerobic exercise training-induced changes in serum adropin level are associated with reduced arterial stiffness in middle-aged and older adults. Am. J. Physiol. - Heart Circ. Physiol. 2015;309:H1642–H1647. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

