NFκB in Pancreatic Stellate Cells Reduces Infiltration of Tumors by Cytotoxic T Cells and Killing of Cancer Cells, via Up-regulation of CXCL12
- PMID: 29909021
- PMCID: PMC6679683
- DOI: 10.1053/j.gastro.2018.05.051
NFκB in Pancreatic Stellate Cells Reduces Infiltration of Tumors by Cytotoxic T Cells and Killing of Cancer Cells, via Up-regulation of CXCL12
Abstract
Background & aims: Immunotherapies are ineffective against pancreatic cancer. We investigated whether the activity of nuclear factor (NF)κB in pancreatic stromal cells contributes to an environment that suppresses antitumor immune response.
Methods: Pancreata of C57BL/6 or Rag1-/- mice were given pancreatic injections of a combination of KrasG12D/+; Trp53 R172H/+; Pdx-1cre (KPC) pancreatic cancer cells and pancreatic stellate cells (PSCs) extracted from C57BL/6 (control) or mice with disruption of the gene encoding the NFκB p50 subunit (Nfkb1 or p50-/- mice). Tumor growth was measured as an endpoint. Other mice were given injections of Lewis lung carcinoma (LLC) lung cancer cells or B16-F10 melanoma cells with control or p50-/- fibroblasts. Cytotoxic T cells were depleted from C57BL/6 mice by administration of antibodies against CD8 (anti-CD8), and growth of tumors from KPC cells, with or without control or p50-/- PSCs, was measured. Some mice were given an inhibitor of CXCL12 (AMD3100) and tumor growth was measured. T-cell migration toward cancer cells was measured using the Boyden chamber assay.
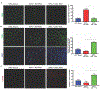
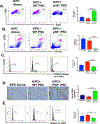
Results: C57BL/6 mice coinjected with KPC cells (or LLC or B16-F10 cells) and p50-/- PSCs developed smaller tumors than mice given injections of the cancer cells along with control PSCs. Tumors that formed when KPC cells were injected along with p50-/- PSCs had increased infiltration by activated cytotoxic T cells along with decreased levels of CXCL12, compared with tumors grown from KPC cells injected along with control PSCs. KPC cells, when coinjected with control or p50-/- PSCs, developed the same-size tumors when CD8+ T cells were depleted from C57BL/6 mice or in Rag1-/- mice. The CXCL12 inhibitor slowed tumor growth and increased tumor infiltration by cytotoxic T cells. In vitro expression of p50 by PSCs reduced T-cell migration toward and killing of cancer cells. When cultured with cancer cells, control PSCs expressed 10-fold higher levels of CXCL12 than p50-/- PSCs. The CXCL12 inhibitor increased migration of T cells toward KPC cells in culture.
Conclusions: In studies of mice and cell lines, we found that NFκB activity in PSCs promotes tumor growth by increasing expression of CXCL12, which prevents cytotoxic T cells from infiltrating the tumor and killing cancer cells. Strategies to block CXCL12 in pancreatic tumor cells might increase antitumor immunity.
Keywords: CXCR4; Chemokine; Cytokine; Immune Suppression.
Copyright © 2018 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Activated pancreatic stellate cells sequester CD8+ T cells to reduce their infiltration of the juxtatumoral compartment of pancreatic ductal adenocarcinoma.Gastroenterology. 2013 Nov;145(5):1121-32. doi: 10.1053/j.gastro.2013.07.025. Epub 2013 Jul 25. Gastroenterology. 2013. PMID: 23891972 Free PMC article.
-
Combination of PD-1 Inhibitor and OX40 Agonist Induces Tumor Rejection and Immune Memory in Mouse Models of Pancreatic Cancer.Gastroenterology. 2020 Jul;159(1):306-319.e12. doi: 10.1053/j.gastro.2020.03.018. Epub 2020 Mar 14. Gastroenterology. 2020. PMID: 32179091 Free PMC article.
-
Galectin-3 Mediates Tumor Cell-Stroma Interactions by Activating Pancreatic Stellate Cells to Produce Cytokines via Integrin Signaling.Gastroenterology. 2018 Apr;154(5):1524-1537.e6. doi: 10.1053/j.gastro.2017.12.014. Epub 2017 Dec 21. Gastroenterology. 2018. PMID: 29274868
-
JTC801 Induces pH-dependent Death Specifically in Cancer Cells and Slows Growth of Tumors in Mice.Gastroenterology. 2018 Apr;154(5):1480-1493. doi: 10.1053/j.gastro.2017.12.004. Epub 2017 Dec 14. Gastroenterology. 2018. PMID: 29248440 Free PMC article.
-
IL-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer.Gut. 2018 Feb;67(2):320-332. doi: 10.1136/gutjnl-2016-311585. Epub 2016 Oct 21. Gut. 2018. PMID: 27797936 Free PMC article.
Cited by
-
Regulation of the tumor immune microenvironment by cancer-derived circular RNAs.Cell Death Dis. 2023 Feb 16;14(2):132. doi: 10.1038/s41419-023-05647-w. Cell Death Dis. 2023. PMID: 36797245 Free PMC article. Review.
-
Cancer-associated fibroblasts as accomplices to confer therapeutic resistance in cancer.Cancer Drug Resist. 2022 Sep 7;5(4):889-901. doi: 10.20517/cdr.2022.67. eCollection 2022. Cancer Drug Resist. 2022. PMID: 36627901 Free PMC article.
-
MicroRNA-200b-3p restrains gastric cancer cell proliferation, migration, and invasion via C-X-C motif chemokine ligand 12/CXC chemokine receptor 7 axis.Bioengineered. 2022 Mar;13(3):6509-6520. doi: 10.1080/21655979.2022.2034585. Bioengineered. 2022. PMID: 35226830 Free PMC article.
-
Cancer-Associated Fibroblasts and Tumor Cells in Pancreatic Cancer Microenvironment and Metastasis: Paracrine Regulators, Reciprocation and Exosomes.Cancers (Basel). 2022 Jan 31;14(3):744. doi: 10.3390/cancers14030744. Cancers (Basel). 2022. PMID: 35159011 Free PMC article. Review.
-
Cancer-Associated Fibroblasts in Pancreatic Ductal Adenocarcinoma: An Update on Heterogeneity and Therapeutic Targeting.Int J Mol Sci. 2021 Dec 14;22(24):13408. doi: 10.3390/ijms222413408. Int J Mol Sci. 2021. PMID: 34948209 Free PMC article. Review.
References
-
- Burnet FM. The concept of immunological surveillance. Prog Exp Tumor Res 1970;13:1–27. - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. - PubMed
-
- Vinay DS, Ryan EP, Pawelec G, et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin Cancer Biol 2015;35 Suppl:S185–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

