Identification of complement inhibitory activities of two chemotherapeutic agents using a high-throughput cell imaging-based screening assay
- PMID: 29909366
- PMCID: PMC6138572
- DOI: 10.1016/j.molimm.2018.06.009
Identification of complement inhibitory activities of two chemotherapeutic agents using a high-throughput cell imaging-based screening assay
Abstract
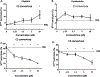
Excessive complement activation contributes significantly to the pathogeneses of various diseases. Currently, significant developmental research efforts aim to identify complement inhibitors with therapeutic uses have led to the approval of one inhibitor for clinical use. However, most existing complement inhibitors are based on monoclonal antibodies, which have many drawbacks such as high costs and limited administration options. With this report, we establish an inexpensive, cell imaging-based high-throughput assay for the large-scale screening of potential small molecule complement inhibitors. Using this assay, we screened a library containing 3115 bioactive chemical compounds and identified cisplatin and pyridostatin as two new complement inhibitors in addition to nafamostat mesylate, a compound with known complement inhibitory activity. We further demonstrated that cisplatin and pyridostatin inhibit C5 convertases in the classical pathway of complement activation but have no effects on the alternative pathway of complement activation. In summary, this work has established a simple, large-scale, high-throughput assay for screening novel complement inhibitors and discovered previously unknown complement activation inhibitory activities for cisplatin and pyridostatin.
Keywords: Complement; complement inhibitor; high-throughput; screening; small compound.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


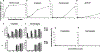
References
-
- Ajona D, Ortiz-Espinosa S and Pio R, 2017. Complement anaphylatoxins C3a and C5a: Emerging roles in cancer progression and treatment. Semin Cell Dev Biol. - PubMed
-
- Blom AM, Volokhina EB, Fransson V, Stromberg P, Berghard L, Viktorelius M, Mollnes TE, Lopez-Trascasa M, van den Heuvel LP, Goodship TH, Marchbank KJ and Okroj M, 2014. A novel method for direct measurement of complement convertases activity in human serum. Clin Exp Immunol 178, 142–53. - PMC - PubMed
-
- Coyle D, Cheung MC and Evans GA, 2014. Opportunity cost of funding drugs for rare diseases: the cost-effectiveness of eculizumab in paroxysmal nocturnal hemoglobinuria. Med Decis Making 34, 1016–29. - PubMed
-
- de Cordoba SR, Tortajada A, Harris CL and Morgan BP, 2012. Complement dysregulation and disease: from genes and proteins to diagnostics and drugs. Immunobiology 217, 1034–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

